003 Note book
Assessments:
Mini exam 1 15% Monday July 29th
Mini exam 2 15% Monday August 19th
Mini exam 3 15% Monday September 2nd
Final Exam 55% Thursday Nov 28th
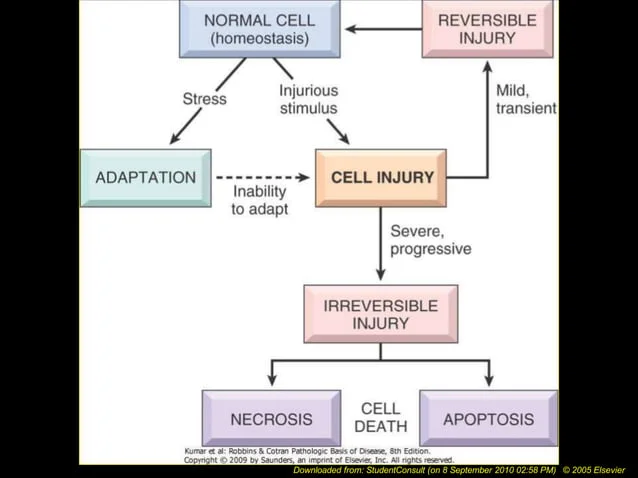
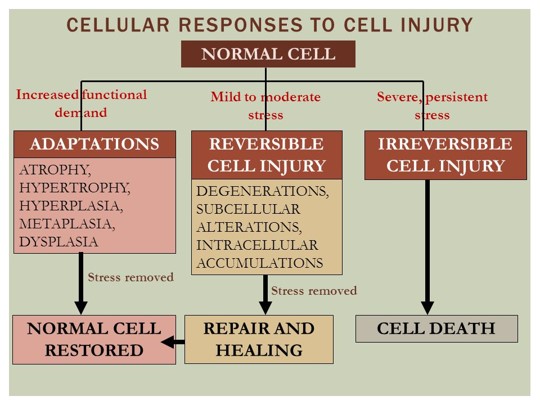
Cell Adaptation
(1) Describe four types of cellular adaptations
(2) List examples of hypertrophy, hyperplasia, atrophy, metaplasia and dysplasia
(3) Compare and contrast apoptosis and necrosis
(4) Define dysplasia and discuss its consequences
(5) Outline common agents that cause cell injury
(1) Describe four types of cellular adaptations
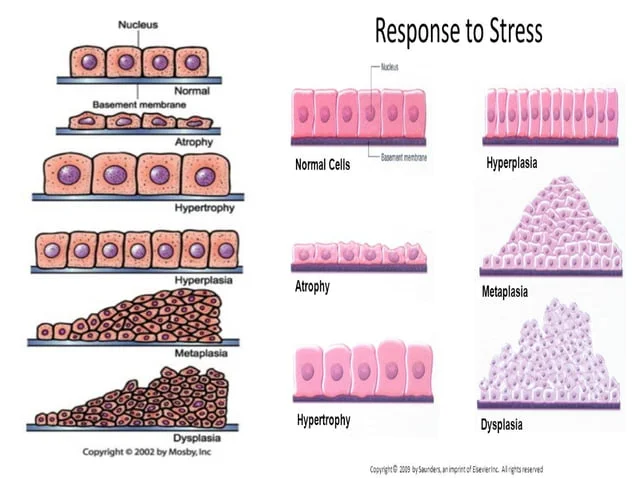
The 4 types of cellular adaptations are:
Hypertrophy
is an increase in cell size
caused by increased functional demand and hormonal stimulation
causes an increase in cell size & cell function
results in an increase in tissue mass due to increased protein synthesis
seen in cardiac, skeletal, and muscle tissue
hyperplasia
is an increase in cell number
occurs as a response to a stimulus and ceases when stimulus is removed
restricted to cells capable of mitosis like the epidermis, intestinal epithelium, and glandular tissue
Common types of hyperplasia: breast enlargement in pregnancy, benign prostatic hyperplasia
atrophy
is a decrease in cell size
due to workload or adverse environmental conditions
is adaptive and reversible
Types:
Disuse atrophy (paralysis)
Degeneration (MS)
Ischaemic atrophy (kidney, heart
Malnutrition atrophy (starvation)
Endocrine stimulation loss (uterine, breast)
metaplasia
change in cell type
reversible replacement of one mature cell type by another (usually a less differentiated cell type)
A response to a persistent irritation and inflammation to cells
May predispose to cancer
Atypical hyperplasia (dysplasia)
Deranged cell growth resulting in mature cells of varying size, shape, and appearance
may be associated with chronic irritation or inflammation
may be reversible if offending agent is removed
Dysplasia is considered a strong precursor of cancer
e.g Cervical cancer
Dysplasia is not a truly adaptive process but is related to hyperplasia
These adaptations allow cells to survive and maintain their function in response to various stimuli or conditions
(2) List examples of hypertrophy, hyperplasia, atrophy, metaplasia and dysplasia
Hypertrophy
Enlarged muscle cells in bodybuilder
Hyperplasia
Breast tissue growth during pregnancy
Atrophy
Muscle wasting in bedridden patients
Metaplasia
Barrett’s esophagus due to acid reflux
Dysplasia
Cervical dysplasia as a precursor to cancer
(3) Compare and contrast apoptosis and necrosis
Apoptosis is programmed cell death, a physiological process eliminating worn-out or damaged cells, while necrosis is cellular death due to injury, causing inflammation and cellular dissolution
Physiological apoptosis is the process that eliminates:
Worn out cells (RBCs)
Cells which have been produced in excess WBCs with infectious response/hepatocytes with hepatitis
Cells which have developed improperly spontaneous abortion
Cells which have genetic damage cancer
Apoptosis involves cell suicide and controlled breakdown of organelles, leading to cellular fragmentation, whereas necrosis in uncontrolled, causing swelling, membrane rupture, and cellular autodigestion
Apoptosis does not trigger inflammation, as the cell contents are contained and phagocytosed, while necrosis leads to inflammation due to the release of cellular contents
(4) Define dysplasia and discuss its consequences
Dysplasia is a condition characterised by abnormal cell growth leading to cells of varying size, shape, and appearance
It is considered a strong precursor of cancer
Consequences of dysplasia include:
an increased risk of developing cancer if left untreated
removing the underlying cause of dysplasia may reverse the condition
Dysplasia is not a truly adaptive process and is often associated with chronic irritation or inflammation
(5) Outline common agents that cause cell injury
Common agents that cause cell injury include:
ischemia
hypoxia
chemical substances
radiation
mechanical factors
These agents can lead to mechanisms of injury such as:
depletion of ATP
mitochondrial damage
entry of calcium into the cell
increase in reactive oxygen species
membrane damage
DNA damage
protein misfolding
Additionally, physical, thermal, and biological factors can also contribute to cell injury
Hepatobiliary
(1) List the risk factors for acute pancreatitis and acute cholecystitis
(2) List the clinical manifestations of acute pancreatitis and acute cholecystitis
(3) Discuss the pathophysiology of acute pancreatitis and acute cholecystitis and how they are related to treatment strategies
(4) Discuss the impact of acute pancreatitis and acute cholecystitis for individuals, family and the society
(1) List the risk factors for acute pancreatitis and acute cholecystitis
The risk factors for acute cholecystitis include:
obesity
middle age
being female
drastic weight loss or acute illness
sickle cell disease
hereditary factors
pregnancy
trauma
On the other hand, the risk factors for acute pancreatitis include:
gallstones
alcohol consumption
infections like Hepatitis B and mumps
certain drugs
endoscopic procedures
trauma
hereditary factors
These risk factors contribute to the development of these conditions
(2) List the clinical manifestations of acute pancreatitis and acute cholecystitis
The clinical manifestations of acute pancreatitis include:
sudden upper abdominal pain that may radiate to the back
Nausea
Vomiting
Fever
Hypotension/Hypovolemia due to increased vascular permeability caused by enzymes
On the other hand, acute cholecystitis typically presents with symptoms such as:
severe right upper quadrant abdominal pain
nausea
vomiting
fever
These symptoms can help healthcare providers in diagnosing and treating these conditions effectively
(3) Discuss the pathophysiology of acute pancreatitis and acute cholecystitis and how they are related to treatment strategies
Acute pancreatitis is characterised by inflammation of the pancreas due to various factors like gallstones or alcohol abuse
this inflammation can lead to the release of digestive enzymes, causing damage to pancreatic tissue and surrounding organs
Treatment includes:
fluid resuscitation to prevent dehydration
antibiotics for infections
surgery in cases of gallstones or infected necrosis
Acute cholecystitis, on the other hand, is inflammation of the gallbladder often caused by gallstones blocking the cystic duct
the treatment involves:
antibiotics
endoscopy for biliary obstruction
surgery to remove gallstones or the gallbladder itself
Both conditions require specific treatments tailored to the underlying causes to manage symptoms and prevent complications
(4) Discuss the impact of acute pancreatitis and acute cholecystitis for individuals, family and the society
Acute pancreatitis and Acute cholecystitis have significant impacts on individuals, families, and society
Individuals may suffer reduced quality of life, weight loss, and potential development of diabetes
Families face pressure and anxiety due to the patient’s recovery period
Societies like NZ have high incidence rates of these conditions, affecting healthcare resources and economic productivity due to hospital stays and loss of income
Acute Abdomen (Peptic/gastric ulcers & Appendicitis)
(1) List the assessment, risk factors and diagnostic tests for peptic ulcer disease and appendicitis
(2) Identify the clinical manifestations of peptic ulcer disease and appendicitis
(3) Discuss the pathophysiology of peptic ulcer disease and appendicitis and how they are related to treatment strategies
(1) List the assessment, risk factors and diagnostic tests for peptic ulcer disease and appendicitis
For peptic ulcer disease, assessment involves endoscopy as the gold standard diagnostic test
Risk factors include:
H. pylori infection
NSAID use
smoking
alcohol consumption
Diagnostic tests include:
endoscopy
testing for H. pylori through stool antigen test
serology
histology
fasting serum gastrin to rule out cancer if multiple or persistent ulcers are present
For appendicitis, assessment includes physical examination checking for rebound tenderness and guarding
Risk factors are unclear but may involve obstruction of the appendix
Diagnostic tests include imaging studies like CT scans or ultrasounds
(2) Identify the clinical manifestations of peptic ulcer disease and appendicitis
The clinical manifestations of peptic ulcer disease include:
abdominal pain
often described as burning or gnawing, that can be relieved by eating or taking antacids
Other symptoms may include
bloating
nausea
vomiting
weight loss
On the other hand, appendicitis typically presents with:
periumbilical pain that shifts to the right lower quadrant (RLQ) as the appendix becomes more inflamed
this pain is accompanied by local tenderness and can progress to peritonitis if the appendix ruptures
(3) Discuss the pathophysiology of peptic ulcer disease and appendicitis and how they are related to treatment strategies
Peptic ulcer disease is caused by injury to the digestive tract by peptic acid, leading to ulcerations in the gastric mucosa.
This can result in ulcerative disorders in the lower esophagus, upper duodenum, and lower stomach
Appendicitis involves obstruction of the appendix leading to bacterial invasion, inflammation, and swelling
Treatment strategies for both conditions focus on reducing acid production for peptic ulcers and typically involve surgical removal of the appendix for appendicitis
Delirium
(1) Describe Delirium (also referred to as acute confusional state)
(2) Identify the clinical manifestations of Delirium and recognize the overlap of acute confusional states
(3) Demonstrate knowledge of how to care for patients with Delirium
(1) Describe Delirium (also referred to as acute confusional state)
Delirium, also known as acute confusional state, is characterized by an acute change in level of conciousness and activity over hours to days
It involves a global change in cognition with inattention, a fluctuating course with disturbances in the sleep-wake cycle and motor control
It is important to differentiate between delirium and dementia, as delirium is often not diagnosed or misdiagnosed, sometimes being attributed to medications or dementia
Delirium presents with clinical manifestations such as:
disordered thinking
euphoria
language impairment
illusions
delusions
hallucinations
reversal of the sleep-wake cycle
inattention
inability to focus
unawareness
disorientation
memory deficits
There is no definitive lab test for diagnosing delirium, so observation and ongoing assessment are crucial
The pathophysiology of delirium involves various mechanisms such as:
depriving the brain of essential substances like oxygen and glucose
toxic effects from drugs
peripheral inflammation triggering changes in the brain’s inflammatory and neurotransmitter functions
physiological and metabolic changes during acute illness
acute psychological stress like pain, discomfort, fear, and sleep disruption
These factors can disrupt the brain’s complex functions, leading to delirium
3 types of Delirium:
Hyperactive delirium is characterized by restlessness, agitation, rapid mood changes, and hallucinations
Hypoactive delirium involves inactivity, reduced motor activity, sluggishness, or abnormal drowsiness
Mixed delirium displays both hyperactive and hypoactive symptoms, with individuals switching between the two states rapidly
Reticular Activating System (RAS)
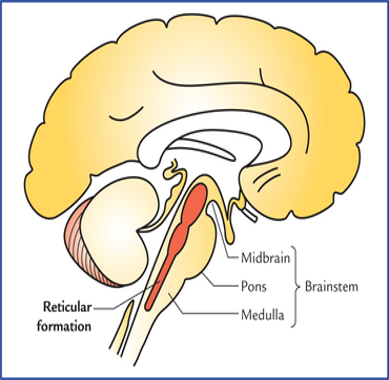
Delirium or ACS arises from disruption of a widely distributed neural network involving the RAS o the upper brainstem.
RAS is located within the thalamus, basal nuclei, specific areas of the cortex, limbic regions & brainstem
(2) Identify the clinical manifestations of Delirium and recognize the overlap of acute confusional states
Clinical manifestations of delirium include an:
acute change in conciousness and activity
global cognitive changes with inattention
fluctuating course affecting sleep-wake cycle and motor control
Delirium can be identified through mnemonic DELIRIUM:
D
Disordered thinking
E
Euphoria
L
Language impairment
I
Illusions/Delusions/Hallucinations
R
Reversal of Sleep-wake cycle
I
Inattention, unable to focus
U
Unawareness, disorientated
M
Memory deficits
It is important to differentiate delirium from other conditions like dementia, as delirium is often misdiagnosed or attributed to other factors
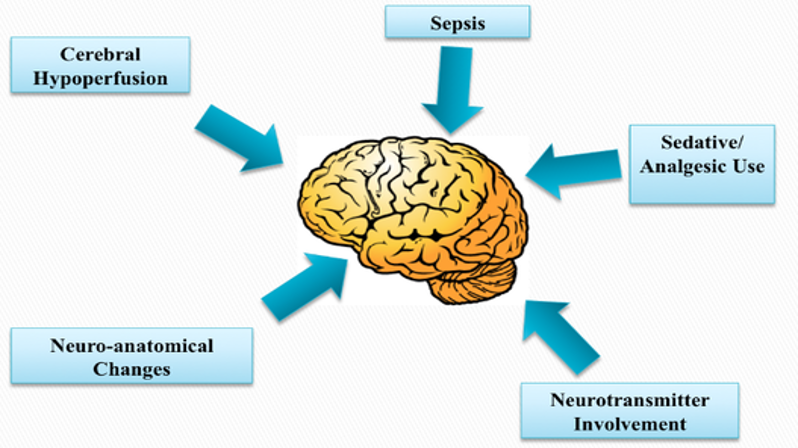
The aetiology of delirium includes various factors such as:
sepsis
has been associated with the development of delirium as well
cerebral hypoperfusion
neuroimaging studies have provided evidence that delirium may manifest as a result of widespread brain dysfunction rather than a localised dysfunction.
it has been suggested that a disruption to cerebral blood flow affecting a large portion of the brain may play a part in the development of delirium
sedative/analgesic use
There has been lot of proposed mechanism of delirium development surrounding sedative and analgesic use
The most common theory involves the use of benzodiazepines which bind GABA receptors in the brain and decrease CNS arousal
This can lead to unpredictable neurotransmission and cerebral functioning resulting in neuronal atrophy and long-term cognitive impairment
neuro-anatomical changes
have been noted in different patient populations experiencing delirium
One study revealed that 61% of critically ill patients were found to have gross white and gray matter lesions or ventricular enlargements
These cellular changes may explain some of the long term cognitive ad behavioural sequelae of delirium
neurotransmitters and hormone involvement
Many different neurotransmitters ad hormones, such as serotonin, catecholamines, cortisol etc. have been suggested to have a part in the development of delirium
Their exact mechanism isn’t very clear in the literature, as both increased and decreased levels of these substances appear to be able to cause delirium
Common causes of delirium can be:
infections (commonly urine or chest)
trauma
surgery
constipation
drug side-effects (e.g opioids or benzodiazepines)
sudden drug withdrawals (e.g antidepressants)
Risk factors for developing delirium include:
advanced age
high comorbidity burden
depression
dementia
frailty
alcohol abuse
benzodiazepine use
(3) Demonstrate knowledge of how to care for patients with Delirium
To care for patients with delirium, it is important to create a quiet, stable, and well-lit environment
Use re-orientation techniques like calendars and family photos, provide explanations during procedures, and reinforce orientation
Avoid physical restraints and ensure correct sensory deficits are addressed
Encourage support from familiar staff and family members
In cases of severe delirium, constant supervision may be necessary to prevent non-compliant behaviour
Additionally, a psych/med review may be needed for managing agitation or aggressive behaviour
Substance Intoxication
(1) Consider the effects of intoxication of substances such as Alcohol, Opioids, and Amphetamines
(2) Outline the processes occurring within the brain during substance intoxication
(3) Identify the harmful effects of overdose and withdrawal from substances
(1) Consider the effects of intoxication of substances such as Alcohol, Opioids, and Amphetamines
Alcohol intoxication can lead to impaired coordination & judgement, and slurred speech
Opioid intoxication can cause euphoria, drowsiness, and decreased respiratory rate
Amphetamine intoxication can result in increased energy, alertness, and decreased appetite
Each substance affects the brain differently, leading to various physical and cognitive effects
Alcohol intoxication:
Reinforcer:
a substance whose pharmacological effects drive the user to continue to use it
Positive reinforcing effects:
gain pleasure
altered conciousness
conform to behaviour of peers
Negative reinforcing effects
relief of stress and negative emotion
relief of withdrawal symptoms
Alcohol (ethanol) absorption
Occurs entire length of digestive tract
skin, lungs, mucous membranes
varies on volume and concentration
food/gastric emptying - first pass if gastric emptying slow
peak levels reached 30-90 minutes
gastric ADH activity
genetic variation
gender
Through body water
differences in body composition and total body water
Ratios based on blood levels (averages)
Blood
Serum - 1:1.18
Brain - 1:0.75
Breath - 2100:1
Saliva - 1:1.12
Metabolism and Excretion
Metabolism rate highly variable
Metabolised at liver, kidney, muscle, lung, intestine, brain (5% excreted unchanged in urine, feces, breath, sweat)
Differences in liver volume, ADH activity
90% ethanol metabolised by ADH
Atleast 6 types encoded by 7 genes
A fast ADH or slow ALDH leads to elevated acetaldehyde levels thereby reducing alcohol drinking
Variations in Ethanol Metabolism
Heavy vs Occasional drinkers
Regular drinkers metabolise alcohol fast than light drinkers as heavy drinkers have more available ADH enzyme
Heavy drinkers generally require a much higher blood alcohol levels to achieve a feeling of intoxication
Male vs Female
Female have proportionally more body fat and less water than males. There Alcohol is dispersed in body water. Women reach intoxication faster than men
Genetics
Acetylaldehyde (ADLH2*2)
is dominant in Chinese, Japanese and Korean descent
Responsible for Alcohol flush reaction
strongly protective against alcohol dependence
Opioid Intoxication
Opioids like opiates act on brain receptors, causing the release of dopamine in the ventral tegmental area and nucleus accumbens.
This leads to effects like:
analgesia
euphoria
drowsiness
detachment from surroundings
relaxation
slurred speech
impaired judgement
Side effects can include:
nausea
vomitting
constipation
drowsiness
constricted pupils
decreased respiratory rate
reduced sexual and aggressive drives
In high doses,
opioids can lead to respiratory depression and potentially death, identified by symptoms like pinpoint pupils, unconciousness, and respiratory depression
Amphetamines
Amphetamine intoxication can lead to various effects on the body
It can cause
euphoria
alertness
excitation
insomnia
grandiosity
dilated pupils
increased heart rate
Clinically it can manifest as cardiovascular issues like:
chest pain
palpitations
hypertension
CNS problems such as:
agitation
violent behaviour
hallucinations
Respiratory symptoms like:
dyspnea
wheezing
Integumentary issues including:
abscesses
lesions
GI problems such as:
abdominal pain
Dental complications like:
tooth decay
peri-dental abscesses
These effects can be harmful and may require medical intervention
(2) Outline the processes occurring within the brain during substance intoxication
Alcohol Intoxication
During Alcohol Intoxication. alcohol modifies membranes in the brain, affecting neurotransmitters like dopamine, glutamate, GABA, and serotonin
It impacts the reward system by interacting with receptors such as DRD2 and NMDA
This alteration in neurotransmitter activity contributes to the pleasurable effects of alcohol consumption
Opioid Intoxication
During substance intoxication, opiates act on opioid receptors in the brain’s ventral tegmental area, leading to the release of dopamine in the nucleus accumbens
This dopamine release results in effects like analgesia, euphoria, drowsiness, and impaired judgement
The substance also causes side effects such as:
nausea
vomiting
decreased respiratory rate
Amphetamine intoxication
during amphetamine intoxication, the drug promotes the release of neurotransmitters like dopamine, serotonin, and norepinephrine in the CNS and PNS nerve endings
It blocks the reuptake of dopamine, leading to euphoric effects in the CNS
This excessive release of neurotransmitters can result in:
heightened alertness
increased energy level
insomnia
dilated pupils
Overtime, tolerance can develop, leading to increased dosages and potential harmful effects on the brain and body
(3) Identify the harmful effects of overdose and withdrawal from substances
Alcohol poisoning:
The most common alcohol poisonings are:
Ethanol - mortality 0.1%
Methanol - mortality 1.0%
Isopropanol - mortality 0.02%
Ethylene glycol - mortality 0.3%
10-14 admissions per 1000 people
Alcohols are the most common accidental toxic ingestions by children younger than 5 years
Treatment:
All alcohols
Larvage - up to 4 hours post ingestion
Activated charcoal
Supportive measures - fluid monitoring, oxygen, airway protection
Methanol/Ethylene Glycol
sodium bicarbonate
Ethanol infusion
Dialysis
Harmful effects of alcohol overdose can include severe intoxication leading to alcohol poisoning, which can result in symptoms like:
confusion
vomiting
seizures
slow breathing
coma or death
Withdrawal from alcohol can lead to symptoms such as:
sudden extreme high blood pressure
tremors
Excite/fear - agitation/irritability
anxiety
hallucinations/confusion - delirium
increased heart rate
seizures
in severe cases, delirium tremens (DT)
For those with alcohol use disorder suddenly stop drinking - they have a spike in glutamate that causes them symptoms common with DT
which is a life-threatening condition characterized by confusion, seizures, and hallucinations and even death as the SNS is in overdrive which can associate to cardiovascular collapse
Opioid overdose
Opioid overdose can lead to:
respiratory depression
unconciousness
pinpoint pupils
Withdrawal from opioids can cause symptoms like:
nausea
vomitting
diarrhea
muscle pain
anxiety
Overdose can be reversed with naloxone, while withdrawal may require medical supervision for management
Acute Cardiac Conditions
(1) Identify the risk factors for acute cardiac conditions
(2) Discuss the pathophysiology of angina, MI, pericarditis, endocarditis & valve disorders
(3) Discuss the clinical manifestations, diagnosis and management of pericarditis, endocarditis & valve disorders
(1) Identify the risk factors for acute cardiac conditions
The risk factors for acute cardiac conditions include non-modifiable like:
advancing age
being male or female after menopause
having family history of coronary artery disease
Modifiable risk factors include:
dyslipidemia
HTN
smoking
diabetes mellitus (DM)
insulin resistance
obesity
sedentary lifestyle
These factors can contribute to conditions like:
acute coronary syndrome
angina
myocardial infarction
pericarditis
endocarditis
valve disorders
(2) Discuss the pathophysiology of angina, MI, pericarditis, endocarditis & valve disorders
Angina is caused by reduces blood flow to the heart muscle due to narrowed arteries
Myocardial infarction (MI) occurs when a coronary artery is completely blocked, leading to heart muscle damage
Pericarditis is inflammation of the pericardium, outer lining of the heart
Endocarditis is an infection or inflammation of the endocardium, often affecting the heart valves
Valve disorders can result from various conditions, such as congenital defects or acquired diseases, leading to improper valve function and potential complications
(3) Discuss the clinical manifestations, diagnosis and management of pericarditis, endocarditis & valve disorders
Pericarditis
Clinical manifestations of pericarditis include:
chest pain
fever
pericardial friction rub
Diagnosis involves:
physical exam
ECG changes
echocardiography
Treatment includes:
NSAIDs
colchicine
corticosteroids
Endocarditis
Endocarditis presents with:
fever
heart murmur
petechiae
Diagnosis requires:
blood cultures
echocardiography
Management involves:
antibiotics
sometimes surgery
Valve disorders
Valve disorders manifest as:
heart murmurs
chest pain
heart failure symptoms
Diagnosis includes:
echocardiography
Treatment may involve:
medications
valve replacement surgery
Acute respiratory conditions
(1) Provide an overview of the structure and aging of the respiratory system
(2) Discuss the pathophysiology, and clinical manifestations of Asthma and other common acute respiratory conditions
(3) Discuss the risks and potential complications of common acute respiratory conditions
(1) Provide an overview of the structure and aging of the respiratory system
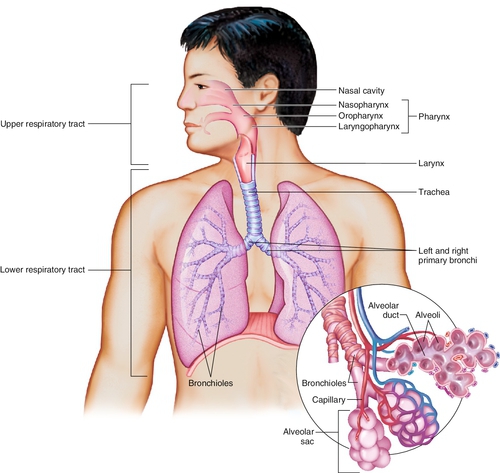
The respiratory system includes structures like:
nasal cavity
pharynx
larynx
trachea
bronchi
bronchioles
alveoli
capillaries for gas exchange
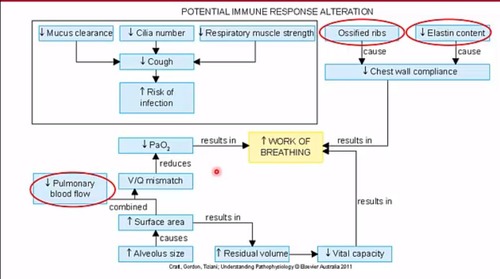
Aging can affect:
immune response
mucus clearance
cilia number
respiratory muscle strength
ribs
elastin content
cough
chest wall compliance
risk of infection
pulmonary function
gas exchange due to changes in these structures
These changes can lead to:
decreased lung functions
reduced vital capacity
increased risk of respiratory conditions like infections and asthma
(2) Discuss the pathophysiology, and clinical manifestations of Asthma and other common acute respiratory conditions & (3) Discuss the risks and potential complications of common acute respiratory conditions
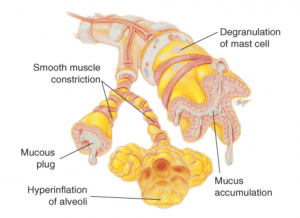
Asthma
it is characterised by intermittent or persistent airway obstruction due to factors like:
bronchial hyperresponsiveness
excess mucus production
atopy
air trapping
this leads to symptoms such as:
wheezing
SOB
chest tightness
coughing
anxiety
Pathophysiological symptoms such as:
edema
mucus
muscle spasms cause resistance to airflow
impairing expiration and leading to air trapping and alveolar hyperinflation
This results in:
uneven ventilation/perfusion
decreased pulmonary blood flow
impaired gas exchange
ultimately, hypoxemia & hypercapnia
Clinical manifestations include:
respiratory distress
increased respiratory rate
use of accessory muscles for breathing
decreased oxygen saturation levels
Asthma diagnosis involves:
history
physical examination
pulmonary function tests
laboratory studies
chest X-ray
Treatment includes:
monitoring lung function
controlling environmental triggers
pharmacologic therapy
patient education with an action plan
Pulmonary Embolism (PE)
occurs when a thrombus dislodges and occludes a pulmonary vessel, leading to decreased blood flow and hypoxia
it commonly arises from deep veins due to factors like:
venous stasis
hypercoagulability
vessel injuries
Symptoms include:
sudden chest pain
dyspnea
tachypnea
tachycardia
anxiety
The obstruction causes:
ventilation-perfusion imbalances
decreased PaO2
pulmonary infarction
HTN
decreased cardiac output
systemic hypotension
shock
PE can be life threatening and requires prompt medical intervention to prevent complications
Atelectasis
is the collapse of lung tissue due to various factors like lack of lung expansion or post-operative complications
there are 2 types:
Absorption
Compression
This condition can lead to:
decreased pulmonary blood flow
impaired gas exchange
respiratory failure
Clinical manifestations may include:
hypoxemia
hypercapnia
Mechanisms of air trapping in atelectasis involve:
issues with air movement during inspiration & expiration
mucus
bronchial plugs
muscle wall collapse
alveolar wall issues
These factors contribute to uneven ventilation/perfusion and decreased alveolar ventilation, which ca result in impaired gas exchange and respiratory failure
Pneumothorax
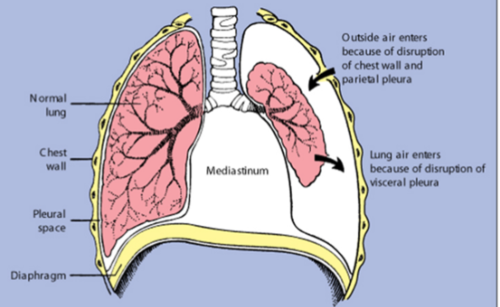
occurs when air enters the pleural space due to a rupture in the pleura
In traumatic cases, like injury, air enters through the chest wall and parietal pleura
This disrupts the pressure balance, leading to lung collapse
Clinical manifestations include:
sudden chest pain
dyspnea
tachypnea
tachycardia
anxiety
Treatment involves:
removing air from the pleural space to re-expand the lung
Pleural effusion
is the accumulation of excess fluid in the pleural space
it can be caused by various conditions like infections, heart failure, or cancer
The pathophysiology involves an imbalance between fluid production and absorption in the pleural space, leading to fluid buildup
This can case symptoms such as:
chest pain
difficulty breathing (dyspnea)
rapid breathing (tachypnea)
fast heart rate (tachycardia)
Diagnosis is usually done through imagine tests like X-rays or ultrasounds
Treatment may involve:
addressing the underlying cause
draining the fluid
medication
Aspiration
occurs when foreign substances are inhale into the respiratory tract
it can lead to:
inflammation
infection
respiratory distress
Pathophysiology involves the entry of substances like food or liquids into the airways, causing irritation, inflammation, and potential blockage
Clinical manifestations include:
coughing
wheezing
chest pain
SOB
in severe cases, aspiration pneumonia
Aspiration can lead to serious complications like lung abscess or respiratory failure if not managed promptly
Treatment involves:
supportive care
antibiotics for infections
bronchoscopy to remove the aspirated material
Pneumonia
is an infection that inflames the air sacs in one or both lungs
it can be caused by bacteria, viruses, or fungi
The pathophysiology involves the invasion of the lung tissue by the infectious agent, leading to an inflammatory response
This response causes the air sacs to fill with pus and other liquid, making it difficult to breathe
Types of pneumonia:
Community-acquired pneumonia
Streptococcus pneumoniae
Mycoplasma pneumoniae
Influenza, Legionella
Hospital-acquired (nosocomial) pneumonia
Staphylococcus aureus by fungi, protozoans
Clinical manifestations include:
cough
fever
chills
difficulty breathing
In severe cases, pneumonia can lead to complications such as respiratory failure
Risk factors for pneumonia include:
age
underlying lung disease
smoking
malnutrition
Treatment usually involves:
antibiotics for bacterial pneumonia
antiviral medications for viral pneumonia
supportive care to relieve symptoms
Bronchiolitis
is a common lower respiratory tract infections, often seen in children under 2 years old
it is mainly caused by the respiratory syncytial virus (RSV)
Clinical manifestations include symptoms like:
runny nose (rhinorrhoea)
cough
poor feeding
labored breathing (dyspnea)
Bronchiolitis is highly contagious
The pathophysiology involves inflammations and obstruction of the small airways in the lungs, leading to symptoms and potential complications
Croup (Acute laryngotracheobronchitis)
is an acute condition affecting the upper airway, commonly seen in children aged 6 months to 5 years
it is often caused by viruses like:
parainfluenza
infleunza A
RSV
The microorganism enters the upper airway, triggering an inflammatory response that leads to swelling and oedema in the upper airway
This swelling can cause upper airway obstruction, resulting in symptoms like a seal-like barking cough
The inflammation and oedema increase resistance to airflow, leading to increased negative pressure in the chest and potential collapse of the upper airway
Clinical manifestations of croup include a:
barking cough, which is distinctive, and the condition is usually self-limiting but may require glucocorticoids to reduce inflammation if severe
Review of the Respiratory System
(1) Review the structure and function of the Respiratory system, related to breathing and respiration and perfusion.
(2) Introduce tests relating to measurement of ventilation
(3) Gain an overview of the development of the respiratory system in the unborn.
(4) Consider the effects of aging on the respiratory system
(1) Review the structure and function of the Respiratory system, related to breathing and respiration and perfusion.
The respiratory system consists of the lungs, airways, and muscles involved in breathing
Air is inhaled through the nose or mouth, travels down the trachea, and enters the lungs through bronchial tubes
In the lungs, oxygen is exchanged for carbon dioxide in tiny air sacs called alveoli
This process is known as respiration
Perfusion, the process of oxygenated blood being delivered to tissues, os facilitated by the respiratory system through the exchange of gases in the alveoli
the diaphragm and intercostal muscles play a crucial role in breathing by expanding and contracting the chest cavity to allow air in and out of the lungs
Overall, the respiratory system ensures the intake of oxygen and removal of carbon dioxide, supporting the body’s metabolic functions
(2) Introduce tests relating to measurement of ventilation
The tests relating to the measurement of ventilation include:
Tidal Volume (TV)
which measures the volume of air breathed in and out during quiet breathing
Vital Capacity (VC)
is the maximum air amount inhaled and exhaled with forced breathing
Forced Vital Capacity
measures the maximum air exhaled forcefully
Forced Expiratory Volume in 1 second (FEV1)
measures the maximum air exhaled in one second
Residual Volume (RV)
is the air volume left in the lungs after forceful exhalation
Total Lung Capacity (TLC)
is the total air amount in maximally expanded lungs, calculated as the sum of RV and VC
These tests provide valuable information about lung function and can help diagnose respiratory conditions
(3) Gain an overview of the development of the respiratory system in the unborn.
The development of the respiratory system in the unborn goes through 5 stages:
Embryonic stage (0-7 weeks)
Psuedogladular stage (7-16 weeks)
Canalicular stage (16-25 weeks)
Saccular stage (25-36 weeks)
Alveolar stage (36 weeks - 6-8 years)
During these stages, the lungs undergo significant growth and maturation, with the alveolar stage being the final stage where the alveoli, responsible fir gas exchange, continue to develop postnatally.
This process is crucial for the unborn to be able to breathe independently after birth
(4) Consider the effects of aging on the respiratory system
Aging affects the respiratory system in various ways
With age, there is a reduction in elastic fibers in the lungs, decreased respiratory muscle strength, and reduced cilia activity
Additionally, there is a decrease in cough efficiency, making older individuals more vulnerable to respiratory infections
The ribs can calcify, the vertebrae can develop osteoporosis, and the alveoli can become “baggy”, leading to decreased lung function
These changes can result in diminished ventilatory response to hypoxia and hypercapnia, making older individuals more susceptible to ventilatory failure or pnuemonia
Nerves triggering coughing become less sensitive, further compromising the respiratory defense mechanisms
Acid/Base Regulation
(1) Review the basics – acids and bases (alkali)
(2) Discuss the role of hydrogen ion concentration in cellular function and dysfunction
(3) Describe how buffering systems help prevent significant fluctuations in pH
(4) Differentiate between respiratory and metabolic acid-base disorders by causes and mechanisms of compensations
(1) Review the basics – acids and bases (alkali)
Acids
are substances that donate protons (H+) when dissolved in water
they can be identified by their sour taste, ability to turn blue litmus paper red, and their corrosive nature
Examples of acids include:
hydrochloric acid (HCl) found in the stomach
Citric acid in citrus fruits
Acetic acid in vinegar
Acids plays a crucial role in various chemical reactions and are essential in many biological processes
Bases
also known as alkalis, are substances that receive protons (H+)
they can neutralize acids by accepting hydrogen ions
Examples of bases include:
metal hydroxides like sodium hydroxide (NaOH) & Potassium hydroxide (KOH)
in the context of cellular function, bases help maintain the pH balance by counteracting the acidic effects of hydrogen ions
This balance is crucial for various cellular processes to function optimally
(2) Discuss the role of hydrogen ion concentration in cellular function and dysfunction
Hydrogen ion concentration plays a crucial role in cellular function and dysfunction
In cellular function,
hydrogen ions are involved in maintaining the normal pH level within cells, which is vital for various cellular to function optimally
for example,
enzymes, which are essential for biochemical reactions in cells, have an optimal pH range for their activity, and any significant deviation in hydrogen ion concentration can affect their function
In cellular dysfunction,
an imbalance in hydrogen ion concentration can lead to acid-base disorders, disrupting cellular activities
For instance,
acidosis, which is characterised by increased hydrogen ion concentration, can interfere with normal cellular functions and lead to serious conditions like hyperkalemia
Therefore, maintaining the balance of hydrogen ions is crucial for proper cellular function and overall health
(3) Describe how buffering systems help prevent significant fluctuations in pH
Buffering systems help prevent significant fluctuations in pH by quickly neutralizing excess acids or bases in the body
The plasma buffer system, respiratory system, and kidneys work together to maintain pH homeostasis
For example,
the respiratory system responds rapidly to pH changes by adjusting CO2 levels
the kidneys, although slower to react, can continue buffering for extended periods by excreting H+ ions and regulating bicarbonate levels
By working in tandem, these systems ensure that pH remains within the normal range, preventing acidosis or alkalosis
(4) Differentiate between respiratory and metabolic acid-base disorders by causes and mechanisms of compensations
Respiratory base disorders are caused by changes in carbon dioxide levels, leading to acidosis (elevated pCO2) alkalosis (low pCO2) due to hypoventilation or hyperventilation, respectively.
Metabolic base disorders result from changes in bicarbonate levels, causing acidosis (reduced HCO3-) or alkalosis elevation of HCO3-) due to non-carbonic acid accumulation or excessive loss of metabolic acids
Compensatory mechanisms involve the kidneys and lungs regulating bicarbonate and carbon dioxide levels to restore pH balance
Respiratory acidosis
is caused by elevated pCO2 due to alveolar hypoventilation, leading to a decrease in pH
The compensation mechanism involves the kidneys retaining bicarbonate (HCO3-) to help normalize pH levels
Metabolic acidosis
is characterised by reduced HCO3- levels or an increase in non-carbonic acids, lowering pH
the compensation mechanism for metabolic acidosis involves the respiratory system increasing ventilation to eliminate carbon dioxide, this raising pH levels
Trauma & Abuse
(1) Understand the impact of adverse childhood events on the individual, whanau and community.
(2) Identify anatomical and pathophysiological changes in child trauma.
(3) Discuss impact of adverse childhood events on adult life
(4) Describe neuroplasticity of the brain
(1) Understand the impact of adverse childhood events on the individual, whanau and community.
Adverse childhood events can have profound impacts on individuals, families (whanau), and communities
Individuals may exhibit behavioural reactions like:
anger
avoidance
anxiety
low confidence
Families can experience:
stress
gried
feelings of failure
Communities may see:
increased violence
aggression
lack of trust
These events can lead to a rang of emotional, psychological, and social challenges that affect the overall well-being of individuals, families, and communities
The long-term effects can include relationships, and even societal problems like crime and substance abuse
It is crucial to address these impacts through support systems, therapy, and community interventions to mitigate and lasting consequences of adverse childhood events
(2) Identify anatomical and pathophysiological changes in child trauma.
Childhood trauma can lead to anatomical and pathophysiological changes in the brain
For example, prolonged exposure to stress hormones like cortisol can impact the development of brain regions involved in emotional regulation and memory, such as the amygdala and hippocampus
These changes can result in alterations in brain structure and function, affecting a child’s ability to cope with stress and regulate emotions
Additionally, trauma can disrupt the formation of neural connections and impact neurotransmitter systems, leading to long-term changes in brain circuitry and functioning
These alterations may contribute to symptoms of anxiety, depression, and other mental health issues commonly seen in individuals who have experienced childhood trauma
(3) Discuss impact of adverse childhood events on adult life
Adverse childhood events can have a significant impact on adult life
Individuals who experience ACEs are at a higher risk of mental and physical illnesses, as well as engaging in dysfunctional behaviours in adulthood
These experiences can lead to difficulties in regulating emotions, forming healthy relationships, and coping with stress
The trauma from childhood can manifest in various ways in adulthood, such as:
increased anxiety
depression
substance abuse
even physical health issues like heart disease or diabetes
Additionally, ACEs can affect cognitive function and decision-making abilities, leading to challenges in work, relationships, and overall well-being
Overall, the impact of adverse childhood events on adult life is profound and can have long-lasting consequences on an individual’s mental, emotional, and physical health
(4) Describe neuroplasticity of the brain
Neuroplasticity refers to the brain’s ability to reorganize itself by forming new neural connections throughout life
this process allows the brain to adapt to new experiences, learn new information, and recover from injuries
involves changes in brain structure, such as global volumetric changes, limbic circuitry, frontal regions, cerebellum, and structural connectivity
It is influenced by both genetics and environmental factors, shaping brain development
For example, trauma can impact brain development by affecting the reptillian brain, limbic system, and neocortex, leading to challenges in cognition, emotional regulation, and survival instincts
Overall, neuroplasticity plays a crucial role in how the brain responds to various stimuli and experiences, highlighting its dynamic and adaptive nature
High Risk Behaviours
(1) Describe the neuroscience of high risk behaviours
(2) Discuss possible pathophysiology of suicide and risk factors
(3) Discuss possible pathophysiology of self harm and risk factors
(1) Describe the neuroscience of high risk behaviours
High-risk behaviours involve actions that can lead to harm or negative consequences
In terms of neuroscience, these behaviours are often linked to the brain’s reward system.
when engaging in high-risk behaviours, the brain’s reward pathways, particularly the release of dopamine, can be activated
This activation reinforces the behaviours, making it more likely to be repeated despite the potential negative outcomes
Additionally, factors like genetics, environment, and past experiences can influence an individual’s propensity for engaging in high-risk behaviours by affecting brain function and decision-making processes
These behaviours can become ingrained due to neuroplasticity, where the brain adapts and changes in response to repeated behaviours
(2) Discuss possible pathophysiology of suicide and risk factors
The possible pathophysiology of suicide involves factors like low levels of brain-derived neurotrophic factor (BDNF) and serotonin,
Low BDNF levels are lined to suicide, major depression, PTSD, schizophrenia, and OCD
Post-mortem studies show reduced BDNF in the hippocampus and prefrontal cortex
Serotonin, a neurotransmitter, is believed to be low in those who die by suicide, with evidence of reduced breakdown product levels in the cerebral spinal fluid
Risk factors for suicide include:
history of depression
anxiety
previous suicide attempts
PTSD
family history
genetic vulnerability
ethnicity
age
poverty
psychosis
knowing someone who died by suicide
These factors, along with demographic, distal, proximal factors, and suicidal ideation, contribute to the complex pathophysiology of suicide
(3) Discuss possible pathophysiology of self harm and risk factors
Self-harm, or Non-Suicidal Self-Injury (NSSI), can be influenced by various risk factors
The possible pathophysiology involves a complex interplay of psychological and biological factors
Individuals may engage in self-harm as a maladaptive coping mechanism to deal with emotional distress, trauma, or mental health issues like anxiety and depression
Isolation, being bullied, and adverse childhood experiences (ACEs) can also contribute to self-harm behaviour
The presence of previous NSSI and exposure to NSSI in peers can normalize and reinforce self-harm tendencies
Additionally, underlying mental health conditions can increase the likelihood of engaging in self-harm as a way to regulate emotions or numb psychological pain
Overall, self-harm can be a manifestation of deeper emotional struggles and a cry for help
Pharmacology in Mental Health
(1) Be able to explain one commonly prescribed medication from each major class of mental health medications
Anxiolytics (Anti-anxiety, Sedatives, Hypnotics)
Alprazolam (Xanax) is benzodiazepine used to treat anxiety disorders
Anti-psychotics (Typical and Atypical)
Aripiprazole (Abilify) is an atypical antipsychotic used to treat schizophrenia and bipolar disorder
Anti-depressants
Sertraline (Zoloft) used to treat depression and anxiety disorders
Stimulants
Methylphenidate (Ritalin) is a common stimulant used to treat attention deficit hyperactivity disorder (ADHD)
(2) Describe the effects on the CNS, indications for use, and Adverse effects and associated risks for:
Anxiolytics (Anti-anxiety, Sedatives, Hypnotics)
Anxiolytics like Benzodiazepine (Diazepam/Valium) act of GABA receptors in the CNS, causing sedation and reducing anxiety by affecting the amygdala in the limbic system
They are used for anxiety and panic disorders, and in alcohol withdrawal
Adverse effects include:
fatigue
drowsiness
muscle weakness
risk of dependence
requiring a long withdrawal period
They are contraindicated in conditions like COPD and liver disease due to potential complications
These medications have CNS depressant effects, are indicated for anxiety-related conditions, and carry risks for side effects and dependency
Anti-psychotics (Typical and Atypical)
Atypical anti-psychotics like Quetiapine (Seroquel) act on CNS receptors for Dopamine and Serotonin, providing a calming effect
they are used for acute and chronic psychosis, schizophrenia and bipolar disorder
Adverse effects include:
increased suicide risk
hypotension
metabolic syndrome exacerbation
dizziness
weight gain
Typical anti-psychotics like Haloperidol (Serenace) at on multiple CNS neurotransmitter receptors, especially Dopamine, leading to extrapyramidal effects
they are indicated for psychosis, schizophrenia, and alcoholic delusions
Adverse effects include:
extrapyramidal effects (movement disorders)
dizziness
constipation
confusion
drowsiness
Anti-depressants
like Fluoxetine (Prozac)
the CNS effects involve inhibiting the reuptake of serotonin, leading to increased serotonin levels in the synaptic space, which helps regulate mood
Indications for use include treating:
depression
anxiety
bulimia nervosa
OCD
premenstrual dysphoric disorder
panic disorder
PTSD
Adverse effects and associated risks may include:
initial increased risk of suicidal thoughts
weight loss
nausea
vomitting
headaches
rashes
dizziness
Stimulants
like amphetamines and methylphenidate
have CNS effects by stimulating neuron activity in excitatory pathways, affecting parts of the brain like the cerebral cortex and limbic region
These drugs are indicated for ADHD treatment
However, they come with adverse effects and risk such as potential:
addiction
insomnia
headache
irritability
nausea
Prolonged use can lead to:
mood changes
depression
agitation
psychosis
These drugs act on neurotransmitters like dopamine & norepinephrine, impacting:
focus
attention
impulse control in individuals with ADHD
(3) Be able to describe the difference between a chemical name, generic name and brand name
The chemical name refers to the exact molecular structure of a drug, providing detailed information about it composition
The generic name is the official name of the drug, usually derived from its chemical name and recognised by health professionals world wide
The brand name is the trademarked name given by the pharmaceutical company marketing the drug
It is unique to that specific company and is used for marketing purposes
For example,
the chemical name for Aspirin is Acetylsalicylic acid, the generic name is Aspirin, and the brand name could be Bayer Aspirin
Acute Diabetic States
(1) The role of the pancreas and hormones insulin and glucagon
(2) Aetiology & cause of diabetes (with a focus on Type 1)
(3) Pathophysiology - the disordered processes and acute complications
(4) The clinical manifestations of acute diabetes states
(1) The role of the pancreas and hormones insulin and glucagon
The pancreas plays a crucial role in regulating blood sugar levels through the secretion of hormones, primarily insulin and glucagon
These hormones work in tandem to maintain homeostasis in the body, particularly concering glucose metabolism
Insulin:
is an anabolic hormone produed by the beta cells of the pancreatic islets
its primary function is to lower blood sugar levels by facilitating the uptake of glucose into cells, especially in the liver, muscle, and adipose tissues
Insulin promotes several key processes:
Glucose uptake, it allows cells to absorb glucose from the bloodstream, which is essential for energy production
Protein Synthesis, insulin encourages the synthesis of proteins, which are vital for growth and repair
Lipid Storage, it aids in the formation and storage of lipids, helping to regulate fat metabolism
Transport of Ions, insulin facilitates the transport of potassium, phosphate, and magnesium across cell membranes, which is important for various cellular functions
In contrast, Glucagon:
is a catabolic hormone produced by the alpha cells of the pancreatic islets
Its primary role is to increase blood sugar levels, particularly during periods of low blood sugar (hypoglycaemia)
Glucagon’s actions include:
Glycogenolysis, it stimulates the conversion of glycogen (stored glucose) in the liver into glucose, which is then released into the bloodstream
Gluconeogenesis, glucagon may promote the conversion of non-carbohydrate sources, such as amino acids and glycerol, into glucose
Lipolysis, it encourages the breakdown of stored fats in adipose tissues, releasing fatty acids into the bloodstream for energy use
Response to stress, the sympathetic nervous system can trigger release during stress, ensuring that energy is available when needed
Together, insulin and glucagon maintain blood sugar levels within a narrow range
(2) Aetiology & cause of diabetes (with a focus on Type 1)
The aetiology of Type 1 Diabetes Mellitus (DM) is multifactorial, involving genetic, immunological and environmental components:
Genetic susceptibility
individuals may have a genetic predisposition to Type 1 DM, often linked to specific genes that influence immune system function
Monogenic Diabetes, caused by mutations in a single gene, can also occur and requires genetic testing for diagnosis
Immune response
Type 1 DM is primarily characterised by an autoimmune response where the body’s immune system mistakenly attacks and destroys the insulin-producing beta cells in the pancreas
This destruction leads to an absolute or significant deficit of insulin, which is critical for glucose metabolism
Environmental factors
various environmental triggers may initiate or exacerbate the autoimmune process
These include:
viral infections, certain viruses have been implicated in triggering the autoimmune response that leads to Type 1 DM
Dietary Factors, for example, exposure to bovine milk in infancy has been suggested as a potential risk factor
Chemical Exposures, certain drugs and chemicals may also play a role in the development of the disease
Pathophysiological changes
the infiltration of lymphocytes and macrophages into the islets of Langerhans in the pancreas results in inflammation and damage to the beta cells
This immune-mediated destruction disrupts insulin production, leading to hyperglycaemia and associated symptoms such as glucosuria (glucose in urine) and diabetic ketoacidosis (DKA), a serious acute complication characterised by the hyperketonemia (high levels of ketones in the blood)
In summary, the aetiology of Type 1 DM involves a complex interplay of genetic predisposition, autoimmune destruction of pancreatic beta cells, and environmental factors that together lead to the clinical manifestations of the disease
(3) Pathophysiology - the disordered processes and acute complications
The pathophysiology of diabtes involves complex disordered processes that lead to acute complications, particularly in individuals with Type 1 Diabetes
Disordered processes
Insulin deficiency, in Type 1 diabetes, the pancreas fails to produce insulin due to autoimmune destruction of beta cells
Insulin in crucial for glucose uptake by cells, and its absence leads to elevated blood glucose levels (hyperglycaemia)
Glucagon overproduction, In response to low insulin levels, glucagon secretion increases. Glucagon promotes gluconeogenesis and glycogenolysis in the liver, exacerbating hyperglycaemia
Metabolic imbalance, the lack of insulin and the presence of glucagon lead to a shift from glucose metabolism to fat metabolism, resulting in the production of ketone bodies. This can lead to diabetic ketoacidosis (DKA)
Acute complications
Hypoglycaemia
this occurs when blood glucose levels drop too low, often due to excessive insulin administration or inadequate food intake
Symptoms include:
confusion
sweating
tremors
can lead to seizures or LOC if untreated
Diabetic Ketoacidosis (DKA)
characterised by high levels of ketones in the blood due to fat breakdown
DKA presents with symptoms such as:
nausea
vomitting
abdominal pain
rapid breathing
fruity-smelling breath
It is a medical emergency requiring prompt treatment with insulin and fluids
Hyperglycaemic Hyperosmolar State (HHS)
This condition is more common in Type 2 diabetes and involves extremely high blood glucose levels without significant ketone production
It leads to severe dehydration and hyperosmolarity, causing confusion, lethargy and can progress to coma
Metformin Associated Lactic Acidosis (MALA)
While primarily associated with Type 2 Diabetes, MALA can occur in patients taking metformin, especially in cases of renal impairment
MALA is characterised by blood lactate levels exceeding 5 mmol/L, indicating significant lactic acidosis
This conditions is a medical emergency due to the potential for severe metabolic disturbances
In summary, MALA results from the interplay of metformin’s pharmacological effects, impaired lactate clearance due to to renal dysfunction, and conditions that promote lactate production, leading to a dangerous accumulation of lactate in the bloodstream
(4) The clinical manifestations of acute diabetes states
The clinical manifestations of acute diabetic states vary depending on the specific condition
Here are the key manifestations for each of the acute complications mentioned:
Hypoglycaemia
this condition occurs when blood glucose levels drop below normal
Clinical manifestations include:
sweating
shakiness or tremors
confusion or irritability
palpitations
hunger
dizziness or lightheadedness
in severe cases, it can lead to seizures or LOC
Diabetic Ketoacidosis
DKA is characterised by the accumulation of ketones due to insufficient insulin
Clinical manifestations include:
Polyuria (increased urination)
Polydipsia (increased thirst)
Nausea & vomitting
Abdominal pain
Fruity-scented breath (due to acetone)
Rapid breathing (Kussmaul respirations)
confusion or altered mental status
Hyperglycaemia Hyperosmolar State (HHS)
this condition is marked by extremely high blood sugar levels without significant ketone production
Clinical manifestations include:
Severe dehydration
polyuria
Polydipsia
confusion or altered consciousness
weakness
visual disturbances
Metformin Associated Lactic Acidosis (MALA)
this rare but serious condition can occur in patients taking metformin, especially in cases of renal impairment
Clinical manifestations include:
lactic acidosis symptoms such as muscle pain or weakness
abdominal discomfort
Rapid breathing
confusion or lethargy
Hypotension (low blood pressure
Each of these acute states presents distinct clinical signs and symptoms that require prompt recognition and management to prevent serious complications
Pathophysiology of wound healing
(1) Review basic anatomy of skin
(2) Describe the 4 phases of wound healing
(3) Identify what is classified as an acute wound
(4) Describe primary and secondary wound healing
(5) Describe factors that affect wound healing & how they impact the individual
(1) Review basic anatomy of skin
The basic anatomy of the skin consists of 3 primary layers: the epidermis, dermis, and subcutaneous tissue (hypodermis)
Epidermis
this is the outermost layer of the skin, primarily composed of keratinized stratified squamous epithelium
It provides a protective barrier against environmental hazards and is responsible for the skin’s pigmentation due to melanocytes
The epidermis is avascular, meaning it does not contain blood vessels, and relies on the dermis for nutrient supply
Dermis
located beneath the epidermis, the dermis is much thicker and contains connective tissue, blood vessels, hair follicles, and various glands (such as sweat and sebaceous glands)
It provides structural support and elasticity to the skin due to the presence of collagen and elastin fibres
The dermis also houses sensory receptors that detect touch, pressure, and temperate
Subcutaneous Tissue (Hypodermis)
this is the deepest later of the skin, consisting of loose connective tissue and fat cells
it act as an insulator, helps regulate body temperature, and serves as an energy reserve
the hypodermis also anchors structures like muscles and bones
Overall, the skin serves multiple serves multiple functions, including protection, temperature regulation, sensation, immune defense, a biochemical processes such as Vitamin D absorption
(2) Describe the 4 phases of wound healing
The wound healing process consists of 4 main phases: Haemostatis Inflammation, Proliferation, and Maturation/Remodelling
Each phase plays a crucial role in the overall healing of damaged tissue
Haemostasis
this is the initial phase that occurs immediately after injury
the primary goal is to stop the bleeding
Blood vessels constrict (vasoconstriction) to reduce blood flow, and platelets aggregate at the site of injury, forming a clot
This clot not only prevents further blood loss but also serves as a temporary barrier against pathogens
Inflammation
following haemostasis, the inflammatory phase begins, lasting for several days
This phase is characterised by the body’s immune response to the injury
White blood cells, particularly neutrophils and macrophages, migrate to the wound site to clear debris and pathogens
This process results in redness, heat, swelling, and pain
The inflammatory response is crucial for preventing infection and setting the stage for tissue repair
Proliferation
this phase typically starts a few days after the injury and can last for weeks
It involves the formation of new tissue
Key processes include angiogenesis (formation of new blood vessels), collagen deposition, and epithelization (regrowth of skin cells
Fibroblasts play a significant role in producing collagen, which provides structural support to new tissue
The wound gradually contract's as myofibroblasts pull the edges together
Maturation/Remodelling
The final phase can last for months to years after the injury
Duing this phase, the newly formed tissue is strengthened and reorganised
Collagen fibers are remodeled, and the wound gains tensile and strength
The scar tissue formed during this phase is usually less vascular and has a fewer cells that the original tissue
The goal is to restore the tissue to its normal function as much a possible
Successful wound healing requires that all 4 phases occur in a coordinated matter
(3) Identify what is classified as an acute wound
An acute wound is classified as a type of injury that generally follows the normal healing trajectory and typically shows signs of healing within a month
Acute wounds are characterised by their ability to progress through the 4 phases of wound healing - haemostasis, inflammation, proliferation, and remodelling - without significant complications
Acute wounds can arise from various cases, including:
Traumatic Wounds
these result from external forces, such as cuts, laceration, or abrasions
For example, a deep cut from a sharp object would be considered an acute wound
Surgical Incisions
wounds created intentionally during surgical procedures are also classified as acute
These incisions are designed to heal in a controlled manner, typically by primary intentions, where the edges of the wound are brought together
Burns
depending on their severity, burns can be acute wounds
First-degree burns may heal quickly, while deeper burns may take longer but still follow the acute healing process
Acute wounds are generally expected heal by primary intention, meaning that the wound edges are approximated and heal with minimal scarring
Factors that can influence the healing of acute wounds include the patient’s overall health, age, nutritional status, and the presence of any underlying conditions
In summary, acute wounds are defined by their timely healing process, typically resolving within one month and progressing though the normal phases of healing, contrasting with chronic wounds that fail to heal in a timely manner
(4) Describe primary and secondary wound healing
Primary and secondary wound healing are two distinct modes of wound healing that differ primarily in the extent of tissue loss and the method by which the wound heals
Primary Intention Healing
this type occurs when there is minimal tissue loss, typically seen in clean, surgical incisions that can be easily sutured
The dermal edges of the wound are closely approximated, allowing for a more straightforward healing process
The benefits of primary intention include reduced scarring and a quicker recovery time
The healing process involves 4 stages:
haemostasis (stopping the bleeding)
inflammation (the body’s response to injury
proliferation (new tissue formation)
maturation/remodeling (strengthening and refining the new tissue)
Because the edges are close together, the healing is efficient, and the risk of infection is lower
Secondary Intention Healing
This method is utilised when there is extensive tissue loss, such as in severe lacerations or large pressure injuries that cannot be sutured
In this case, the wound edges are not approximated, and healing must occur from the base of the wound upward
This process is more prolonged and complex, as it involves the formation of granulation tissue and the eventual contraction of the wound
Secondary intention healing often results in increased scarring due to the larger area of tissue that must regenerate and the longer healing time
The 4 stages of healing still apply, but the process may be less predictable, and wounds can progress backward or forward based on various internal and external factors affecting the patient
In summary, primary intention is characterised by minimal tissue loss and quick healing with less scarring, while secondary intention involves significant tissue loss, longer healing times, and typically more pronounced scarring
(5) Describe factors that affect wound healing & how they impact the individual
Wound healing is influenced by a variety of factors that can either promote or hinder the healing process
Understanding these factors is crucial for effective patient care
Here are some key factors affecting wound healing:
Bacterial Infection
the presence of bacteria can lead to infection, which prolongs the inflammatory phase and can result in delayed healing or chronic wounds
Infections can cause increased inflammation, tissue damage, and can lead to systemic complications
Wound Dehiscence
this refers to the reopening of a wound, often due to inadequate healing or excessive tension on the wound edges
Dehiscence can lead to further complications, including infection and prolonged recovery time
Necrosis
the presence of dead tissue (necrotic tissue) in a wound can impede healing by providing a medium for bacterial growth and delaying the formation of new tissue
Debriding may be necessary to remove necrotic tissue and promote healing
Elevated Blood Glucose Levels (BGL)
high BGL, commonly seen in diabetic patients, can impair the immune response and reduce the efficiency of the healing process
It can lead to poor circulation and neuropathy, which further complicates wound healing
Nosocomial Infections
These are infections acquired in a healthcare setting
They can significantly impact wound healing by introducing resistant bacteria, leading to complications that can delay recovery and increase healthcare costs
Other factors
Additional factors include age, nutritional status, oxygenation, underlying health conditions 9like diabetes or vascular diseases), medications (such as corticosteroids) and lifestyle choices (like smoking)
for instance, older adults may experience slower healing due to reduced cellular regeneration, while adequate nutrition (especially protein and vitamins) is essential for tissue repair
In summary, the interplay of these factors can significantly affect the wound healing process, influencing the individual’s recovery time, risk of infections, and overall health
Acute Kidney Injury
(1) Review the anatomy and physiology of the Urinary and Renal Systems
(2) Differentiate between Pre-Renal, Intra-Renal &Post-Renal causes of acute kidney injury.
(3) Identify exemplars of Acute Kidney Injury including Tubular Necrosis and Nephrotoxicity
(4) Recognise the impact of AKI on the individual and the community
(1) Review the anatomy and physiology of the Urinary and Renal Systems
The urinary and renal systems are crucial for maintaining homeostasis, regulating fluid balance, and excreting waste products from the body
Anatomy
kidneys
there are two kidneys, located on either side of the spine, with the left kidney typically positioned slightly higher than the right
Each kidney contains:
renal cortex : the outer layer where filtration occurs
renal medulla : the inner layer, consisting of renal pyramids and collecting ducts
renal pelvis : the funnel-shapes structure that collects urine before it moves to the ureter
renal columns and papillae : structurs that separate the renal pyramids and direct urine into the calyx
Nephron
the functional unit of the kidney, approximately one million per kidney, consists of:
Bowman’s Capsule : encloses the glomerulus, where filtration begins
Glomerulus : a network of capillaries that filter blood
Proximal Convoluted tubule : reabsorbs water, ions, and nutrients
Loop of Henle : creates a concentration gradient for urine concentration
Distal Convoluted tubule : further adjusts the composition of urine
Collecting duct : collects urine from multiple nephrons and transports it to the renal pelvis
Ureters
two tubes that transport urine from the kidneys to the bladder
Bladder
a muscular sac that stores urine until excretion
Urethra
the tube through which urine is expelled from the body
Adrenal glands
located top each kidney, these glands produce hormones that regulate metabolism, immune response, and blood pressure
Physiology
the physiology of the urinary and renal systems is centred around the kidneys, which are vital organs responsible for filtering blood, regulating fluid balance and excreting waste products through urine
Kidney structure:
each kidney contains approximately one million functional nephrons
A nephron consists of several key components:
glomerulus
bowman’s capsule
proximal convoluted tubule
Loop of Henle
distal convoluted tubule
collecting duct
The renal cortex contains the glomeruli an proximal tubules, while the renal medulla houses the Loop of Henle and collecting ducts
Filtration
blood enters the kidneys through the renal arteries, which branch into smaller arterioles leading to the glomeruli
Here, blood is filtered under pressure, allowing water, electrolytes, and small molecules to pass into Bowman’s capsule while retaining larger molecules like proteins and blood cells
Reabsorption
as the filtrate moves through the proximal convoluted tubule, essential substances such as glucose, amino acids, and ions are reabsorbed back into the bloodstream
The Loop of Henle further concentrates urine by reabsorbing water and sodium, creating a concentration gradient in the medulla
Secretion
in the distal convoluted tubule, additional waste products and excess ions are secreted into the filtrate from the blood, helping to maintain electrolyte balance and pH levels
Excretion
the final urine, which contains waste products, excess water, and electrolytes, is collected in the renal pelvis and transported to the bladder via the ureters
The bladder stores urine until it is excreted through the urethra
Regulation
the kidneys also play a crucial role in homeostasis by regulating blood pressure through the renin-angiotensin-aldosterone system, maintaining acid-base balance, and controlling electrolyte levels
Hormones such as erythropoietin and renin, produced by the kidneys, further contribute to these regulatory functions, with EPO stimulating red blood cell production and renin playing a key role in blood pressure regulation through the RAS system
(2) Differentiate between Pre-Renal, Intra-Renal &Post-Renal causes of acute kidney injury.
Acute Kidney Injury (AKI) can be categorised into 3 main types based on the underlying causes: Pre-Renal, Intra-renal, and Post-renal
Pre-Renal AKI
this type occurs due to factors that reduce blood flow to the kidneys, leading to ischemia
common causes include:
dehydration
heart failure
severe blood loss
the kidneys are structurally normal, but their function is impaired due to inadequate perfusion
Intra-Renal AKI
this type involves direct damage to the kidney tissue itself
the most common cause is Acute Tubular Necrosis (ATN)
which can result from ischemia or exposure to nephrotoxins such as certain antibiotics and contrast media used in imaging studies
Intra-Renal AKI reflects structural damage, often seen in hospitalised patients
Post-Renal AKI
this type arises from obstruction in the urinary tract that impedes urine flow, leading to increased pressure in the kidneys
Causes can include:
kidney stones
tumors
enlarged prostate
The obstruction can occur at any point in the urinary system, from the kidneys to the urethra
In summary, Pre-Renal AKI is due to reduced blood flow, Intra-Renal AKI is due to direct kidney damage, and Post-Renal AKI is due to obstruction in the urinary tract
(3) Identify exemplars of Acute Kidney Injury including Tubular Necrosis and Nephrotoxicity
Acute Kidney Injury (AKI) can be exemplified by conditions such Acute Tubular Necrosis (ATN) and Nephrotoxicity
Acute Tubular Necrosis (ATN)
is the most common cause of intrarenal AKI, characterised by damage to the kidney’s tubular cells
this damage can occur due to two primary factors:
Ischaemia, refers to reduced blood flow to the kidneys which can happen in situations like severe dehydrations or shock
Nephrotoxins, are substances that can harm the kidney tissue, including certain medications (like some antibiotics) and contrast media used in imaging studies
The significance of ATN lies in its prevalence, especially among hospitalised patients, indicating a critical area for monitoring and intervention
Nephrotoxicity
refers to the toxic effects on the kidneys caused by various substances
this can include drugs (e.g non-steroidal anti-inflammatory drugs, certain antibiotics, and chemotherapy agents) and environmental toxins
Nephrotoxic agents can lead to cellular injury and death in the renal tubules, contributing to the development of AKI
The recognition of nephrotoxicity is crucial for preventing AKI, especially in patient’s with pre-existing kidney conditions or those receiving high-risk medications
Both ATN and nephrotoxicity highlight the importance of early detection and management of AKI, as they can significantly impact an individual’s health and the broader community by increasing healthcare costs and the burden on medical resources
Early intervention can improve outcomes and reduce the long-term effects of kidney damage
(4) Recognise the impact of AKI on the individual and the community
Acute Kidney Injury (AKI) has significant impacts on both individuals and communities, affecting health outcomes, healthcare systems, and economic stability
Impact on the individual
Health consequences
AKI is associated with a rapid decline in renal function, leading to the retention of metabolic wastes, which can cause symptoms like:
fatigue
confusion
fluid overload
The mortality rate exceed 30%, indicating a severe risk to life
Quality of life
individuals may experience complications such as chronic kidney injury disease (CKD) or require dialysis, leading to a diminished quality of life
Symptoms of AKI can lead to hospitalisation, which disrupts daily activities and responsibilities
Psychosocial Effects
the stress of dealing with a serious health condition can lead to anxiety and depression
Patients may also face stigma or fear regarding their health status, impacting their social interactions and mental well-being
Impact on the community
Healthcare system Burden
AKI contributes to increased healthcare costs due to hospital admissions, prolonged stays, and the need for specialised treatment like dialysis
This can strain healthcare resources, particularly in regions with limited medical facilities
Economic impact
the economic burden extends beyond healthcare costs, as individuals may be unable to work during recovery, leading to lost wages and decreased productivity
this can have a ripple effect on local economies
Public health concerns
high rates of AKI can indicate broader public health issues, such as inadequate access to healthcare, environmental factors, or prevalent diseases
Addressing these underlying causes is essential for community health improvement
In summary, AKI poses serious health risks for individuals, leading to potential long-term complications and psychological distress
For communities, it represents a significant burden on healthcare systems and economic stability, necessitating comprehensive strategies for prevention, early detection, and management
Infectious Diseases
(1) Refresh your knowledge of common pathogens and how the immune system works against infectious diseases.
(2) Understand the complex interactions between humans and microorganisms
(3) Describe types of infectious organisms and the types of diseases they cause
(4) Identify who is at risk for infectious diseases
(5) Discuss how infectious agents cause damage to the body
(1) Refresh your knowledge of common pathogens and how the immune system works against infectious diseases.
Common pathogens include bacteria, viruses, fungi, and parasites, each capable of causing various infectious diseases
Bacteria
these are sing-celled organisms that can reproduce independently
some bacteria are beneficial, but pathogenic bacteria can cause diseases such as strep throat, tuberculosis, and urinary tract infections
The immune system combats bacterial infections primarily through the action of antibodies and phagocytic cells, which engulf and destroy bacteria
Viruses
are much smaller than bacteria and require a host cell to replicate
they can cause diseases such as influenza, HIV/AIDS, and COVID-19
The immune response against viruses involves both humoral immunity (antibody production) and cell-mediated immunity, where cytotoxic T cells recognise and destroy infected cells
Fungi
these can be unicellular (like yeast) or multicellular (like molds)
Fungal infections, such as athlete’s foot and candidiasis, often affect individuals with weakened immune systems
the immune system responds to fungi through the activation of T cells and the production of specific antibodies
Parasites
these organisms live on or in a host and can cause diseases such as malaria and giardiasis
the immune response to parasites is complex and often involves both innate and adaptive immunity, including the production of antibodies and the activation of eosinophils
The immune system works against these pathogens through various mechanisms:
Innate immunity
this is the first line of defense and includes physical barriers (like skin), chemical barriers (like stomach acid), and immune cells (like macrophages and neutrophils) that respond quickly to infections
Adaptive immunity
this is a more specific response that develops over time
it involves the activation of lymphocytes (B & T cells)
B cells produce antibodies that specifically target antigens (substances from pathogens)
T cells can directly kill infected cells or help coordinate the immune response
In summary, common pathogens include bacteria, viruses, fungi, protozoa, and prions, each capable of causing various diseases upon invading the body and multiplying. The immune system defends against these infectious agents through a complex network of cells and mechanisms. It identifies and targets pathogens using innate immunity, which provides immediate but non-specific responses, and adaptive immunity, which develop specific responses tailored to particular pathogens. This dual approach enables the immune system to recognise, attack, and eliminate invading microorganisms while also remembering past infections to mount faster responses in future encounter
(2) Understand the complex interactions between humans and microorganisms
The complex interactions between humans and microorganisms encompass a dynamic relationships that can lead to both beneficial and harmful outcomes
These interactions are influenced by various factors, including the type of microorganisms, the host’s immune response, and environmental factors
pathogen invasion
microorganisms such as bacteria, viruses, fungi, protozoa, and prions can invade the human body
infection occurs when these pathogens multiply and produce disease, often causing harm to the host
for instance, bacteria can cause localised infections like strep throat or systemic infections such as sepsis
Immune response
the human immune system plays a crucial role in defending against these pathogens
it recognises and responds to foreign invaders through innate and adaptive immunity
Innate immunity provides immediate defense through barriers (like skin) and immune cells, while adaptive immunity develops a targeted response to specific pathogens, creating memory cells for faster responses in future encounters
Microbiome Interactions
not all microorganism are harmful; many are beneficial and form part of the human microbiome
these beneficial microbes help in digestions, synthesize vitamins, and protect against pathogenic organisms by competing for resources and space
The balance between beneficial and harmful microorganisms is critical for maintaining health
Environmental factors
the interactions are also influenced by environmental factors such as sanitation, nutrition, and healthcare access
Poor sanitation can facilitate the spread of infectious diseases, while good nutritions can enhance immune function
Evolving pathogens
pathogens can evolve rapidly, developing resistance to treatments and vaccines
this evolution can lead to the emergence of new diseases or the resurgence of previously controlled infections, a complicating the human-microbe relationship
Risk factors
certain populations are at higher risk for infectious diseases, including the immunocompromised, elderly, and those with chronic conditions
Understanding these risk factors in essential for public health strategies to prevent and control infections
In summary the complex interactions between humans and microorganism involve a dynamic and evolving relationship where pathogens - such as bacteria, viruses, fungi, protozoa, and prions - invade the human body, multiply and potentially cause disease
This interaction is not merely adversarial; it encompasses a range of responses from the human immune system, which works to combat these infections can vary, leading to localised infections, disseminated infection, or systemic diseases that can harm the host.
Understanding these interactions is crucial for identifying at-risk populations and developing effective strategies to maintain to manage an
(3) Describe types of infectious organisms and the types of diseases they cause
Infectious organisms, also known as pathogens can be categorised into several types, each associated with specific diseases
Bacteria
these are single-celled organism that can cause a variety of diseases
Three major types of bacteria are:
Cocci, spherical bacteria, which can lead to infections such as strep throat (caused by Streptococcus) and toxic shock syndrome (associated with Staphylococcus)
Bacilli, rod-shaped bacteria, responsible for diseases like tuberculosis (Myobacterium tuberculosis) and pnuemonia (various bacterial strains)
Spirilla, spiral-shaped bacteria, which can cause diseases such as syphillis (Treponema pallidum)
Viruses
these are much smaller than bacteria and require a host cell to repicate
Viral infections can lead to diseases such as infleuenza, HIV/AIDS, and COVID-19
Fungi
these organisms can be single-celled (like yeast) or multicellular (like molds)
Fungal infections can cause conditions such as athlete’s foot, ring worm, and systemic infections in immunocompromised individuals
Protozoa
These are singe-celled organisms that can cause diseases such as malaria (caused by Plasmodium species) and giardiasis (caused by giardia lamblia)
Prions
these are infectious proteins that ccan lead to neurodegenerative diseases such as Creutzfeldt-Jakob disease and mad cow disease (BSE)
Each of type of pathogen interacts with the host’s immune system differently, leading to various disease manifestations
For instance, bacterial infections often involve the production of toxins that damage tissues, while viral infections may hijack host cells for replications, leading to cell death
Understanding these interactions is crucial for developing effective treatments and preventive measures against infectious diseases
(4) Identify who is at risk for infectious diseases
Individuals at risk for infectious diseases can be categorised based on several factors, including age, health status, lifestyle, and environmental conditions
Here are some key groups:
Young children and infants
their immune systems are still developing, making them more susceptible to infections
Vaccination schedules are critical for protecting this group
Elderly Individuals
older adults often have weakened immune systems due to age-related decline in immune function, chronic illnesses, or medications that suppress immunity
Individuals with Chronic diseases
people with conditions such as diabetes, heart disease, or HIV/AIDS are at higher risk because their immune systems may be compromised or less effective at fighting infections
Immunocompromised individuals
this includes those undergoing chemotherapy, organ transplant recipients, or individuals on immunosuppressive medications
their bodies are less capable of defending against pathogens
Pregnant women
pregnancy can alter immune responses, making women more vulnerable to certain infections that can also affect fetal health
Healthcare workers
they are frequently exposed to infectious agents due to their work environment, increasing their risk of contracting diseases
Travelers
individuals who travel to areas with endemic diseases or poor sanitation may be at risk of infectious not common in their home countries
Individuals with poor nutrition
malnutrition can impair immune function, making individuals more susceptible to infections
People living in overcrowded for unsanitary conditions
high population density and inadequate sanitation can facilitate the spread of infectious diseases
Substance abusers
those who use intravenous drugs or engage in risky sexual behaviours may be at increased risk for infections like HIV or hepatitis
Understanding these risk factors is crucial for implementing preventive measures and targeting innterventions effectively
As highlighted in the objectives, recognising who is at risk helps in managing and controlling the spread of infectious diseases
(5) Discuss how infectious agents cause damage to the body
Infectious agents cause damage to the body through various mechanisms, which can be broadly categorised based on the type of pathogen involved - bacteria, viruses, fungi, protozoa, and prions
Each type of pathogen has unique methods of causing harm:
Bacteria
can cause damage tissues directly by invading cells and multiplying, leading to cell lysis (bursting) and inflammation
they may also produce toxins that disrupt normal cellular functions
for example, some bacteria release exotoxins that can interfere with nerve function or enterotoxins that can affect the GI tract, causing symptoms like diarrhea
Viruses
invade host cells and hijack their machinery to replicate
this often results in cell death, either through direct lysis of the cell or by triggering apoptosis (programmed cell death)
The immune response to viral infections can also cause tissue damage, as the body attempts to eliminate the infected cells
Fungi
Fungal infections can cause damage through the release of enzymes that break down host tissues, leading to inflammation and necrosis
some fungi can also produce mycotoxins, which can have systemic effects on the body, impacting organs and causing severe illness
Protozoa
Protozoan parasites can invade and destroy host cells, leading to tissue damage
they often evade the immune system and can cause chronic infections, leading to ongoing inflammation and damage to organs, as seen in diseases like malaria
Prions
are misfolded proteins that induce abnormal folding of normal proteins in the brain, leading to neurodegenerative diseases
this results in progressive damage to neural tissue, causing severe neurological symptoms and ultimately death
Overall, the damage caused by infectious agents can manifest as localised symptoms (like redness and swelling), systemic effects (such as fever and malaise), and long-term complications (like organ failure or chronic disease). The immune system’s response to these pathogens is crucial in determining the extent of damage caused by infectious agents. When a pathogen invades the body, the immune system activates various mechanisms to counteract the infection.
This response can lead to localised symptoms, such as redness and swelling, which are often signs of inflammation as the body directs immune cells to the site of infection
In addition to localised responses, the immune system can trigger systemic effects, including fever and malaise
These symptoms are part of the body’s broader response to infection, often aimed at creating an environment less favorable for pathogens and signaling the need for rest and recovery
Furthermore, if the immune response is inadequate or if the infection is particularly severe, long-term complications may arise. These can include organ failure or the development of chronic diseases, as the body may suffer lasting damage from the infection or from an overactive immune response that attacks healthy tissues.
Overall, the immune system plays a pivotal role in managing infections, balancing the need to eliminate pathogens while minimizing harm to the host
Understanding these interactions is essential for recognising who is at risk for infectious diseases and for developing effective treatment strategies
Traumatic Brain Injury
(1) Revise relevant neuroanatomy and physiology
(2) Outline general mechanisms of neuronal injury
(3) Discuss the aetiology, pathophysiology, clinical manifestations, diagnosis and treatment methods for decreased level of consciousness and brain injury
(1) Revise relevant neuroanatomy and physiology
Relevant neuroanatomy and physiology encompass the structure and function of the nervous system, which is divided into the Central Nervous system (CNS) and the Peripheral Nervous system (PNS):
Central Nervous system (CNS)
this includes the brain and spinal cord
the brain is responsible for processing sensory information, controlling motor functions, and facilitating cognitive processes
It consists of various regions such as the cerebrum, cerebellum, and brainstem, each with distinct functions
The spinal cord serves as a conduit for signals between the brain and the rest of the body, and it also mediates reflex actions
Peripheral Nervous System (PNS)
this system comprises cranial and spinal nerves that extend from the CNS to the rest of the body.
it is further divided into the autonomic nervous system (ANS) and the somatic nervous system
The ANS regulates involuntary bodily functions and is subdivided into the sympathetic and parasympathetic systems, which control the body’s fight-or-flight response and rest-and-digest activities, respectively
The somatic nervous system controls voluntary movements by innervating skeletal muscles
Neuronal physiology
neurons are the fundamental units of the nervous system, responsible for transmitting information through electrical impulses
They consist of a cell body, dendrites (which receive signals), and an axon (which sends signals)
Neurotransmitters facilitate communications between neurons at synapses. The physiology of neurons involves mechanisms such as action potentials, synaptic transmission, and plasticity, which are crucial for learning and memory
Understanding these components is essential for discussing neuronal injury, as damage to any part of this system can lead to various clinical manifestations, including decreased levels of consciousness and brain injury
(2) Outline general mechanisms of neuronal injury
General mechanisms of neuronal injury caa
(3) Discuss the aetiology, pathophysiology, clinical manifestations,
Diagnosis and treatment methods for decreased level of consciousness and brain injury can be categorised into several key types:
Traumatic Injury
this includes physical damage to neurons caused by external forces, such as in concussions or to her head injuries
Ischaemic injury
this occur when there is a reduction in blood flow to the brain, leading to a lack of oxygen (hypoxia) and nutrients necessary for neuronal survival
Excitation
excessive stimulation of neurons can lead to excitotoxicity, where neurons become damages due to overactivation, often seen in conditions like seizures
Pressure
increased intracranial pressure can compress brain tissue, disrupting normal neuronal function and potentially leading to cell death
Environmental alterations
various factors that disrupt the stable environment of neurons can cause injury
This includes:
Hypoxia - lack of oxygen
Electrolyte imbalance - disruption in ion concentrations can affect neuronal excitability
Hypoglycaemia - insufficient glucose supply impairs energy metabolism in neurons
Acidosis/Alkalosis - pH imbalances can adversely affect neuronal function
High temperature - elevated body temperature can lead to cellular stress and damage
Sepsis - systemic infections can lead to inflammatory responses that harm neuronal tissue
These mechanisms can lead to primary injuries, which may include complications like raised intracranial pressure and decreased cerebral blood flow, ultimately resulting in further neuronal damage and dysfunction
Transmission of pain
(1) Define and consider the role of pain
(2) Review the different types of pain
(3) Explore the physiology of pain and pain pathways
(4) Define pain perception & methods of modulation of pain
(1) Define and consider the role of pain
Pain serves a crucial role in the human nervous system, functioning as an essential mechanism for survival.
It acts as a warning system that alerts us to actual or potential injury, prompting immediate attention and action to prevent further harm.
This protective aspect of pain is vital; without it, individuals might not recognise dangerous situations or injuries, leading to more severe damage
According to the International Association for the Study of Pain (IASP), pain is defined as “an unpleasant and emotional experience with actual or potential tissue damage.”
This definition highlights the complexity of pain, which is not merely a physical sensation but also involves emotional and psychological components
Pain can vary widely in its experience, being highly subjective and influenced by individual factors such as past experiences, cultural background, and psychological state
The role of pain extends beyond mere detection of harm; it also motivates behavioural changes
For instance, if someone touches a hot surface an feels pain, the immediate rection is to withdraw the hand thereby preventing further injury
This immediate response underscores pain’s function as a protective mechanism, ensuring that the body can respond quickly to threats. Moreover, pain perception can be modulated through various methods, including pharmacological interventions, psychological therapies, and physical treatments
Understanding the physiology of pain and its pathways is essential for developing effective pain management strategies, which can enhance quality of life for individuals suffering from chronic pain conditions
In summary, pain is a fundamental aspect of human experience, serving as a critical alert system that protects us from harm, motivates us to take action, and is influenced by a myriad of physiological and psychological factors
(2) Review the different types of pain
Nociceptive pain
this type of pain arises from actual or potential tissue damage and is typically a response to noxious stimuli
it is characterised by the activation of inflammatory processes and is considered a normal biological response
Nociceptive pain is generally acute and resolves once the underlying tissue heals
It responds well to typical analgesics
Somatic pain
this is a subtype of nociceptive pain that is well-localised and can be felt in the skin, tissues, and muscles
it is often described as sharp, aching, or throbbing
for example, a cut on the skin or muscle strain would produce somatic pain, which is easily identifiable and localised to the affected area
Visceral pain
in contrast, visceral pain is poorly localised and originates from internal organs
It is often described as dull, cramping, or colicky and may be associated with additional symptoms such as nausea or sweating
An example of visceral pain could be the discomfort felt during a gallbladder attack, which may be referred to other areas of the body, making it harder to pinpoint
Understanding these classifications is crucial for effective pain management and treatment strategies, as different types of pain may require different approaches for relief
(3) Explore the physiology of pain and pain pathways
The physiology of pain involves a complex interplay of various components within the nervous system, primarily through specialised nerve endings known as nociceptors
These nociceptors are the first-order neurons that detect harmful stimuli, such as thermal, mechanical, or chemical insults, and convert these signals into electrical impulses
Once activated, nociceptors transmit pain signals via their axons to the spinal cord, specifically through the dorsal horn
Here, they synapse with second-order neurons, which are part of the spinothalamic tract. This tract carries the pain signals upward to the brain. The spinothalamic neurons ascend through the spinal cord and brainstem, ultimately reaching the thalamus, where they synapse with third-order neurons
The thalamus acts as a relay station, processing and forwarding the pain information to the cerebral cortex, where pain is consciously perceived and interpreted
In the cerebral cortex, different areas are involved in the perception of pain, including the somatosensory cortex, which helps localise the pain, and the anterior cingulate cortex and insula, which are associated with the emotional aspects of pain
Pain modulation can occur at various levels of this pathway. For instance, descending pathways from the brain can influence the transmission of pain signals at the spinal cord level, either enhancing or inhibiting the perception of pain
This modulation can involve neurotransmitters such as endorphins, which can reduce the perception of pain, or other chemicals that may amplify it.
In summary, the pain pathway involves a series of neurons from nociceptors to the cerebral cortex, where pain is processed and perceived
Understanding this pathway is crucial for developing effective pain management strategies
(4) Define pain perception & methods of modulation of pain
Pain perception is the process by which the nervous system interprets pain signals
It involves the activation of pain pathways in the nervous system, which can be influenced by various factors, including tissue injury and inflammation
When tissue is damaged, excitatory neuromodulators such as substance P, glutamate, and somatostatin are released, enhancing the sensation of pain
Conversely, inhibitory neuromodulators like GABA, glycine, serotonin, norepinephrine, and endorphins work to dampen the pain signals, providing a balance in pain perception
Methods of modulation of pain can be categorised into pharmacological and non-pharmacological approaches
Pharmacologically, medications can target the excitatory or inhibitory pathways. For instance, opioids like beta-endorphins and enkephalins bind to opioid receptors, providing significant pain relief by enhancing inhibitory neuromodulation
Other medications may include non-steroidal anti-inflammatory drugs (NSAIDs) that reduce inflammation and thus decrease the release of excitatory neuromodulators
Non-pharmacological methods can include physical therapy, cognitive-behavioral therapy, acupuncture, and mindfulness can also modulate pain perception by addressing the psychological and physical aspects of pain
In summary, pain perception is a complex interplay of physiological processes and subjective experiences, with various methods available for modulation, ranging from medications to
Musculoskeletal & Spinal Cord Injury
(1) Provide a review of muscle fibres and the types of muscle tissue.
(2) Introduce types of muscle damage.
(3) Provide an overview and definitions for types of fractures.
(4) Review the process of fracture healing and consider complications that can occur.
(5) Review types of spinal injury and how damage at varying levels impact on functioning
(1) Provide a review of muscle fibres and the types of muscle tissue.
Muscle fibers, also known as myocytes, are specialised contractile cells that play a crucial role in movement by generating force through contraction
These cells are rich in mitochondria, which provide the necessary ATP for energy during muscle activity.
There are 3 primary types of muscle tissue:
Skeletal muscle
this the of under voluntary control and is responsible for the movement of bones and the body
skeletal muscle fibres are striated and can contract rapidly but tire easily
they are typically attached to the skeleton and are involved in activities such as walking, running, and lifting
Cardiac muscle
found exclusively in the heart, cardiac muscle is involuntary and striated, similar to skeletal muscle
however, cardiac muscle fibres are interconnected, allowing for synchronised contractions that pump blood throughout the body
this type of muscle is highly resistant to fatigue due to its continuous activity
Smooth muscle
this type is also involuntary and non-striated
Smooth muscle fibres are found in the walls of hollow organs such as the intestines, blood vessels, and bladder
They contract more slowly than skeletal muscle and can sustain contractions for longer periods, facilitating functions like digestion and blood flow regulation
In summary, muscle fibers are essential for movement, and the three types of muscle tissue - skeletal, cardiac, and smooth - each serve distinct functions in the body, contributing to overall mobility and physiological processes
(2) Introduce types of muscle damage.
muscle damage can occur in various forms, primarily categorised into strains, sprains, and avulsions
Strain
this refers to a tear or injury to a muscle or tendon
strains typically occur when a muscle is stretched beyond its limits or forced to contract too strongly
the severity of a strain can vary from mild overstretching to complete tears, which can significantly impact muscle function and strength
Sprain
a sprain involves a tear or injury to a ligament, which is the tissue that connects bones at a joint
Sprains can affect the stability of joints and range of motion, leading to pain, swelling, and difficulty in movement
like strains, sprains can range from mild (slight stretching) to severe (complete tears)
Avulsion
an avulsion is a more severe form of injury where a tendon or ligament completely separates from its bony attachment site
This type of injury often requires surgical intervention for repair and can lead to significant functional impairment if not treated properly
In addition to these types of damage, the repair process for skeletal muscle involves unique mechanisms
Skeletal muscle fibers cannot divide like other cells but can undergo hypertrophy, where they enlarge by laying down new protein
The process of satellite cells, which are mononucleated quiescent cells located beneath the basal lamina, plays a crucial role in muscle repair
when muscle damage occurs, these satellite cells can divide slowly and, after division, fuse with existing muscle fibers to help regenerate and repair the damaged tissue
However, their capacity to repair is limited, particularly in cases of severe damage
Understanding these types of muscle damage is essential for effective treatment and rehabilitation strategies, as each type may require different approaches to healing and recovery
(3) Provide an overview and definitions for types of fractures.
Fractures are classified based on their characteristics and the nature o the break in the bone
Here’s an overview of the main types of fractures:
Complete vs Incomplete
Complete fracture
the bone is broken all the way through, resulting in two separate pieces
Incomplete fracture
the bone is partially broken meaning it may be cracked but not fully separated
Closed vs Open
Closed (simple) fracture
the fracture does not penetrate the skin, meaning there is no external wound
Open (compound) fracture
the broken bone breaks through the skin, creating an open wound and increasing the risk of infection
Comminuted fracture
this type involves the bone being shattered into three or more pieces, often resulting from high-impact trauma
Linear fracture
a fracture that runs parallel to the long axis of the bone, typically seen in long bones
Oblique fracture
this fracture occurs at an angle across the bone, often resulting from a twisting or bending force
Spiral fracture
similar to an oblique fracture but caused by a twisting force, resulting in a spiral-shaped break
Transverse fracture
a straight break across the bone, which is typically caused by a direct blow or stress
Understanding these classifications is crucial for diagnosis and treatment as the type of fracture can influence the healing process and the approach to management
Treatment often involves reduction (realigning of the bone) and immobilization (using casts or splints) to allow proper healing
(4) Review the process of fracture healing and consider complications that can occur.
The process of fracture healing involves several stages, beginning immediately after the fracture occurs.
Initially, a haematoma forms at the fracture site due to blood vessel damage, creating a blood-filled swelling. This haematoma serves as a foundation for the healing process
Next, fibrocartilage is laid down to form a soft callus, which splints the broken bone. Phagocytes play a crucial role in this phase by removing cellular debris, while fibroblasts deposit collagen to stabilise the fracture. This soft callus is eventually replaced by a bony callus made of spongy bone through a process called endochondral ossification
The bony callus is then remodeled over time. The spongy bone is gradually replaced by compact bone, resulting in a permanent patch that restores the bone’s strength and structure
However, complications can arise during this healing process. Improper reduction or immobilization of the fracture can lead to several issues:
Nonunion
this occurs when the bone ends do not heal together, resulting in a persistent fracture
Delayed union
the healing process takes longer than expected, which can be due to factors like inadequate blood supply, infection, or poor nutrition
Malunion
this happens when the bone heals in an incorrect position, leading to deformity or functional impairment
In summary, fracture healing is a complex process involving stages of haematoma formation, callus development, and remodeling
Proper treatment and monitoring are essential to ensure effective healing and minimize complications
(5) Review types of spinal injury and how damage at varying levels impact on functioning
Spinal injuries can be categorised based on the nature of the injury and the level of the spinal cord affected
The types of spinal injuries include:
Cord concussion
a temporary disruption of spinal cord function without structural damage
symptoms may resolve completely
Cord contusion
bruising of the spinal cord, which can lead to varying degrees of neurological deficits depending on the severity
Cord compression
pressure on the spinal cord, often due to vertebral injuries or herniated discs, which van impair function below the injury site
Cord laceration
a cut or tear in the spinal cord, leading to significant loss of function and potential permanent damage
Cord Transection
complete severing of the spinal cord, resulting in total loss of function below the injury level
The impact of spinal cord damage varies significantly depending on the level of the injury:
Cervical Injuries (C1-C8)
these injuries can lead to quadriplegia, affecting all our limbs and potentially impairing respiratory function if the injury is high (C1-C3)
Thoracic Injuries (T1-T12)
these typically result in paraplegia, affecting the legs and lower body
Individuals may retain arm function but lose control over bowel, bladder, and sexual functions
Lumbar Injuries (L1-L5)
these can also result in paraplegia, with varying degees of leg function
individuals may retain some hip and knee movement but may have difficulty with walking
Sacral injuries (S1-S5)
these injuries usually affect the pelvic organs and lower limbs, leading to issues with bowel and bladder control, but may allow for some leg movement
In summary, the level and type of spinal injury directly correlate with the extent of functional loss, impacting mobility, autonomic functions, and overall quality of life
Allergy & Anaphylaxis
(1) Revise the 5 classes of antibodies
The 5 classes of antibodies, also known as immunoglobins, are distinguished by their structure, function, and the type of immune response they mediate:
IgM, this is the first antibody produced during the primary immune response to an antigen
it is typically found in the bloodstream and is effective in formula complexes with antigens, leading to their destruction
IgA, predominantly found in mucosal areas, such as saliva, tears, and secretions, IgA plays a crucial role in mucosal immunity
it helps protect body surfaces that are exposed to foreign substances
IgD, this class functions primarily as a receptor on B cells, helping to initiate the B cell’s activation and the subsequent immune response
its exact role in serum is less understood compared to other immunoglobulins
IgG, the most abundant antibody in the bloodstream, constituting about 80-85% of immunoglobulins, IgG is vital for long-term immunity
it can cross the placenta, providing passive immunity to the fetus
IgE, this antibody is primarily involved in allergic reactions and defense against parasitic infections
it binds to allergens and triggers histamine release from mast cells and basophilis, leading to allergic symptoms
Each class of antibody has unique characteristics that enable it to perform specific roles in the immune response, contributing to the body’s defense against pathogens and foreign substances
(2) Define the terms allergen and hypersensitivity
An allergen is a specific type of antigen that triggers and exaggerated immune response, leading to hypersensitivity reactions in susceptible individuals
Allergens can be derived from various environmental sources, such as pollens, moulds, foods, animal dander
when a person is exposed to an allergen, their immune system may react inappropriately, resulting in inflammation and tissue damage
This response can manifest in different ways, including immediate reactions to proteins or complex organic materials, or delayed reactions to simpler inorganic substances like metals
Hypersensitivity refers to the inappropriate or exaggerated immune response to an allergen
it is categorised into different types based on the mechanism of the immune response and the timing of the reaction
for example, immediate hypersensitivity occurs rapidly after exposure to the allergen, often within minutes, while delayed hypersensitivity may take hours or even days to develop
The immune system’s response can lead to various symptoms, ranging from mild (such as sneezing or skin rashes) to severe (such as anaphylaxis, a life-threatening reaction)
In summary, allergens are specific antigens that provoke hypersensitivity reactions, which are inappropriate immune responses that can cause a range of symptoms and health issues
Understanding these terms is crucial for identifying and managing allergic reactions effectively
(3) Define and describe anaphylaxis
Anaphylaxis is a severe and potentially life-threatening allergic reaction that occurs rapidly, often within minutes of re-exposure to an allergen
It is characterised by a systemic response that can affect multiple body systems simultaneously
The primary features of anaphylaxis include:
Symptoms
the reaction is marked by significant edema (swelling) and bronchoconstriction, which can lead to difficulty breathing
In severe cases, swelling of the throat may occur, potentially blocking the airway and resulting in respiratory distress
Other symptoms may include hives, swelling of the face or lips, abdominal pain, nausea, and vomiting
Cardiovascular effects
Anaphylaxis can cause a dramatic drop in blood pressure due to widespread vasodilation (the widening of blood vessels) and increased permeability of blood vessels, leading to fluid leakage
This can result in shock and collapse if not treated promptly
Mechanism
the underlying mechanism of anaphylaxis involves IgE-mediated hypersensitivity
when an individual is exposed to an allergen, IgE antibodies is exposed to an allergen, IgE antibodies bind to mast cells, triggering the release of histamine and other inflammatory mediators
this leads to the rapid onset of symptoms
Treatment
the immediate treatment for anaphylaxis is the administration of adrenaline (epinephrine), typically delivered via an EpiPen
this medication acts quickly to reverse the symptoms by constricting blood vessels, increasing blood pressure, and dilating airways, thus alleviating respiratory distress
Prevention and Management
individuals with known severe allergies are often advised to carry an EpiPen and to avoid known allergens
education on recognizing the early signs of anaphylaxis is crucial for timely intervention
In summary, anaphylaxis is a critical medical emergency that requires immediate recognition and treatment to prevent severe complications or death
(4) Define and describe other types of hypersensitivity reactions
Hypersensitivity reactions are exaggerated or inappropriate immune responses to antigens that can lead to disease or damage in the host
There are 4 main types of hypersensitivity reactions:
Type I - IgE mediated
this reaction is characterised by the production of Immunoglobulin E (IgE) antibodies in response to an allergen
Upon re-exposure to the allergen, IgE binds to mast cells and basophils, leading to the release of histamines and other mediators
This can cause immediate allergic reactions such as hay fever, asthma, and anaphylaxis, which is a severe systemic response that can occur when allergens enter the bloodstream
Type II - Tissue-specific Reactions
in this type, antibodies (usually IgG or IgM) bind to antigens on the surface of specific cells, leading to cell destruction through mechanisms such as complement activation or phagocytosis
Clinical examples include autoimmune hemolytic anemia and Goodpasture syndrome, where the immune system mistakenly targets its own tissues
Type III - Immune Complex Mediated
this reaction occurs when immune complexes (antigen-antibody complexes) are formed and deposited in tissues, leading to inflammation and tissue damage
this can activate the complement system and recruit inflammatory cells
Conditions such as systemic lupus erythematosus and rheumatoid arthritis are examples of type III hypersensitivity
Type IV - Cell Mediated
as mentioned in the excerpt, this type involves T lymphocytes and macrophages
it is a delayed response, meaning symptoms may take hours or days to manifest
T cells recognise specific antigens and initiate an immune response, which can result in inflammation and tissue damage
Clinical examples include contact dermatitis (e.g., poison ivy), tuberculosis skin test reactions, and graft rejection.
Each type of hypersensitivity has distinct mechanisms and clinical manifestations, highlighting the complexity of the immune system’s response to perceived threats
Shock States
(1) Identify the various types of shock leading to impaired perfusion
There are several types of shock that lead to impaired perfusion each with distinct causes and mechanisms
Cardiogenic Shock
this type occurs due to ineffective cardiac pumping, often resulting from conditions like myocardial infarction or severe heart failure
The heart’s inability to pump blood effectively leads to decreased cardiac output and inadequate tissue perfusion
Hypovolaemic shock
this is caused by a significant decrease in blood volume, which can be further categorised into:
Hemorrhagic shock
resulting from severe blood loss, such as from trauma or surgery
Non-hemorrhagic shock
caused by fluid loss from other sources, such as severe dehydration, burns, or gastrointestinal losses
Septic shock
this type arises from massive systemic vasodilation due to severe infections, leading to a drop in blood pressure and inadequate perfusion to organs
the body’s inflammatory response to infection causes blood vessels to dilate excessively
Neurogenic shock
this occurs due to a loss of sympathetic tone, often following spinal cord injuries
it results in vasodilation and decreased vascular resistance, leading to hypotension and impaired perfusion
Anaphylactic shock
this is a severe allergic reaction that causes widespread vasodilation and increased vascular permeability, leading to a rapid drop in blood pressure and impaired organ perfusion
Understanding these types of shock is crucial for identifying risk factors, recognising clinical manifestations, and developing appropriate treatment strategies.
Each type has unique pathophysiological mechanisms that can lead to severe consequences if not promptly addressed, including organ damage and systemic inflammation
(2) List the risk factors for types of shock
The risk factors for types of shock, particularly hypovolemic shock, include:
Severe bleeding
this can be either internal or external and leads to significant fluid loss
Major or multiple fractures or major trauma
these conditions can result in substantial blood loss
Severe burns or scalds
such injuries can cause fluid loss from the body
Severe diarrhoea and vomiting
both can lead to dehydration and a decrease in blood volume
Severe sweating and dehydration
excessive fluid loss through sweat can contribute to hypovolemic shock
These factors highlight the critical nature of maintaining fluid volume in preventing hypovolemic shock and its associated complications
(3) Discuss pathophysiology of shock states and how they are related to treatment strategies
The pathophysiology of shock states involves a complex interplay of physiological responses aimed at maintaining tissue perfusion and oxygenation when faced with a critical reduction in blood flow
Shock can be categorised into several types, including hypovolaemic, cardiogenic, distributive, and obstructive shock, each with distinct underlying mechanisms
In hypovolaemic shock, which is charaterised by a significant decrease in intravascular volume (typically over 15%), the body initiates compensatory mechanisms to restore perfusion
These include sympathetic nervous system activation, which increases heart rate and contractility, and the renin-angiotensin-aldosterone system (RAAS), which promotes fluid retention and vasoconstriction
Antidiuretic hormone (ADH) is also released to conserve water.
However, if the shock state persists, these compensatory mechanisms can fail, leading to decreased blood pressure, vascular fluid shifts, and impaired organ perfusion, ultimately resulting in acidosis and systemic inflammation
The treatment strategies for shock states are closely related to their pathophysiology For hypovolemic shock, the primary treatment involves fluid resuscitation to restore intravascular volume and improve cardiac output
This can include crystalloids or colloids, depending on the severity and cause of the shock. In cases of cardiogenic shock, where the heart’s ability to pump is compromised, treatment may involve medications to improve cardiac contractility or mechanical support devices
Distributive shock, such as septic shock, often requires vasopressors to counteract vasodilation and restore systemic vascular resistance
Understanding the pathophysiology of shock is crucial for developing effective treatment strategies. For instance, recognising that hypovolaemic shock results from volume loss informs the need for rapid fluid replacement
Similarly, understanding the role of systemic inflammation in septic shock can guide the use of antibiotics and other supportive therapies
Overall, timely and appropriate interventions based on the underlying mechanisms of shock can significantly improve patient outcomes
(4) Discuss the impact of shock states for individuals, family, and the society
Shock states have profound impacts on individuals, families, and society, stemming from their physiological, psychological, and economic consequences
Impact on Individuals
for individuals, experiencing shock can lead to severe health complications, including organ failure, prolonged hospitalisation, and even death
the immediate effects of shock, such as hypotension and impaired organ perfusion, can result in acute symptoms like confusion, lethargy, and respiratory distress
Long-term consequences may include chronic health issues, reduced quality of life, and mental health challenges such as anxiety or depression due to the trauma of the experience and potential loss of independence
Impact on families
Families of individuals in shock states often face emotional and financial strain
The stress of a loved one’s critical condition can lead to anxiety and emotional distress among family members
Additionally, the need for caregiving and support during recovery can disrupt family dynamics and responsibilities
Financially, families may incur high medical costs, especially if prolonged treatment or rehabilitation is necessary, which can lead to economic hardship or debt
Impact on Society
On a societal level, shock states contribute to a significant burden on healthcare systems
increased hospital admissions, extended lengths of stay, and the need for specialised care can strain resources and lead to higher healthcare costs
This can affect insurance premiums and public health funding
Furthermore, the loss o productivity due to illness or disability can impact the workforce, leading to economic losses
Public health initiatives aimed at preventing shock states, such as education on risk factors and early intervention strategies, become essential to mitigate these impacts
In summary, the effects of shock states extend beyond the individual, affecting families emotionally and financially, while also placing a considerable burden on societal resources and healthcare systems
Addressing these impacts requires a comprehensive approach that includes medical treatment, psychological support, and community resources
 Knowt
Knowt