Electrolysis
Key vocabulary
Electrolysis – decomposition of an ionic compound (molten or in solution) using electricity.
Electrolyte – molten salts or solutions of acids, bases, or salts that conduct electricity and are broken down by electrolysis.
Electrode – metal or graphite rods connected to a power supply, allowing current through the electrolyte.
Anode – positive electrode; attracts anions (negative ions).
Cathode – negative electrode; attracts cations (positive ions).
Anions – negatively charged ions moving to the anode.
Cations – positively charged ions moving to the cathode.
Molten – the liquid form of a heated solid above its melting point.
Aqueous – dissolved in water.
ELECTROLYSIS
There are two ways a substance conducts electricity:
By mobile electrons
By mobile ions
Substance | Conducts electricity? | Why? |
|---|---|---|
Magnesium (s) | Yes | Mobile electrons |
Calcium chloride (s) | No | Ions are fixed in the solid lattice |
Sodium bromide (aq) | Yes | Mobile ions in aqueous solution |
Carbon dioxide (g) | No | No mobile electrons or ions |
Graphite (s) | Yes | Mobile electrons in delocalised system |
Metals, graphite, and molten/aqueous ionic compounds conduct electricity as they have mobile electrons or ions, without involving a chemical reaction.
Covalent compounds do not normally conduct electricity due to the absence of free electrons or ions.
Some covalent substances conduct in solution, as they ionise in water (e.g. HCl → H⁺ + Cl⁻).
Ionic compounds do not conduct in solid state — ions are fixed.
Ionic compounds conduct when molten or aqueous because ions are free to move.
Electrolysis of Molten Ionic Compounds
In this demonstration, you’ll observe how electricity can break down a compound—lead(II) bromide—when it’s molten.
Safety:
This is done in a fume cupboard due to toxic bromine gas.
Method :
Set up the circuit as shown.
Gently heat the lead(II) bromide until it melts.
Observe any changes during electrolysis.
Turn off the power and stop heating.
Note any final observations.
Diagram:
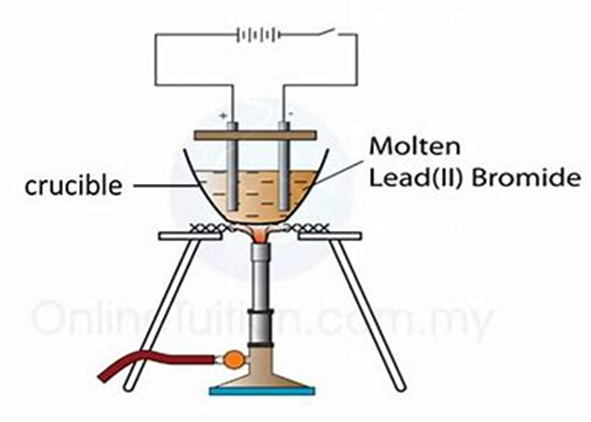
Answers:
Why is a lamp included in the circuit?
To show if current is flowing—the lamp lights up when the circuit is complete and the electrolyte conducts.Why is graphite used for the electrodes?
Graphite is a good conductor, chemically inert, and withstands high temperatures.Does the bulb light when the lead(II) bromide is solid? Explain.
No—it doesn't light because the ions are locked in place and can't move to carry charge.Does the bulb light when the lead(II) bromide is molten? Explain.
Yes—molten lead(II) bromide has mobile ions that allow current to pass.What observation did you make at one of the electrodes? What is the name given to this electrode?
A grey metal forms at the cathode (negative electrode)—this is lead.What is seen when the molten lead(II) bromide is poured away?
A pool of molten grey lead remains at the bottom of the crucible.What happens to the lead(II) bromide in this experiment?
It is decomposed into lead and bromine by the electric current.What evidence is there that it is the electric current and not the heat that causes the changes to the lead(II) bromide?
Molten lead(II) bromide only breaks down when the current flows, not just from heating alone.What is the meaning of the word electrolysis?
Electrolysis is the breakdown of an ionic compound using electricity.
Extension – Half Equations (with state symbols):
At the cathode (reduction):
Pb²⁺(l) + 2e⁻ → Pb(l)At the anode (oxidation):
2Br⁻(l) → Br₂(g) + 2e⁻
Conclusion:
At the cathode (negative electrode)
• Pb²⁺ ions (cations) are attracted to the cathode where they gain electrons and are reduced to lead.
• Pb²⁺(l) + 2e⁻ → Pb(l)
The lead ions are said to be discharged.
At the anode (positive electrode)
• Br⁻ ions (anions) are attracted to the anode where they lose electrons and are oxidised to bromine.
• 2Br⁻(l) → Br₂(g) + 2e⁻
The bromide ions are also said to be discharged.
The electrons released at the anode travel through the external circuit to the cathode, where they reduce lead ions.
Overall reaction:
PbBr₂(l) → Pb(l) + Br₂(g)
Molten lead(II) bromide is thus broken down by electric current into lead metal and bromine gas.
• The reactions at the electrodes are written as ionic half-equations.
• Positive ions go to the cathode (-), gain electrons, and are reduced.
• Negative ions go to the anode (+), lose electrons, and are oxidised.
Oxidation is loss of electrons.
Reduction is gain of electrons.
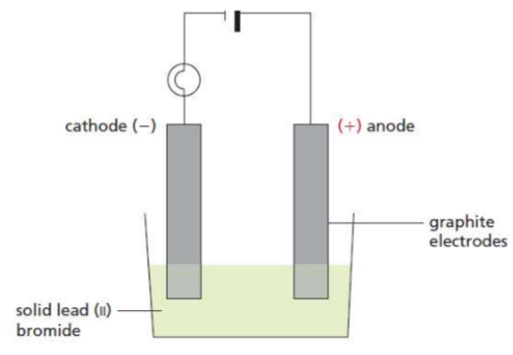
Electrolysis of molten electrolytes
Electrolyte | Ions present | Half equation at the anode | Half equation at the cathode |
|---|---|---|---|
lead(II) iodide, PbI₂ | Pb²⁺, I⁻ | 2I⁻ → I₂ + 2e⁻ | Pb²⁺ + 2e⁻ → Pb |
sodium chloride, NaCl | Na⁺, Cl⁻ | 2Cl⁻ → Cl₂ + 2e⁻ | Na⁺ + e⁻ → Na |
aluminium oxide, Al₂O₃ | Al³⁺, O²⁻ | 2O²⁻ → O₂ + 4e⁻ | Al³⁺ + 3e⁻ → Al |
magnesium bromide, MgBr₂ | Mg²⁺, Br⁻ | 2Br⁻ → Br₂ + 2e⁻ | Mg²⁺ + 2e⁻ → Mg |
• Reduction occurs at the cathode.
• Oxidation occurs at the anode.
Electrolysis of Aqueous Solutions
The electrolysis of aqueous solutions is more complex than molten compounds.
Take sodium chloride as an example:
• As a molten compound, it contains two ions:
Na⁺ and Cl⁻
• As an aqueous solution, it contains four ions:
Na⁺, Cl⁻, H⁺, and OH⁻
This is because water itself partially ionises to give H⁺ and OH⁻ ions, which compete with the ions from the solute during electrolysis.
Safety:
This experiment will be carried out in a fume cupboard.
A U-tube is filled with sodium chloride solution and a few drops of UI solution added.
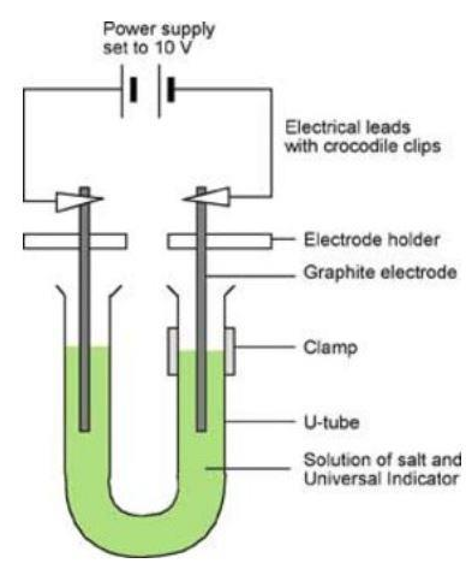
What do you observe during the experiment? What can we infer from these observations?
Effervescence at both electrodes. Gas produced at both electrodes
Solution containing universal indicator started green as the solution is neutral
When the power is turned on the indicator turned purple suggesting that the solution was turning alkaline
The purple color of the indicator becomes colorless at the anode.
What can this tell you about the species discharged or those left remaining after electrolysis?
The positive ion discharged must be hydrogen, rather than sodium as a gas was produced at the cathode
The ion causing the solution to become alkaline is hydroxide
This must means that the negative ion discharged was chloride and the OH- ions remained in solution
Ions present:
Na⁺, Cl⁻, H⁺, OH⁻
Cathode reaction:
2H⁺(aq) + 2e⁻ → H₂(g)
Anode reaction:
2Cl⁻(aq) → Cl₂(g) + 2e⁻
As H⁺ and Cl⁻ ions are discharged during electrolysis, Na⁺ and OH⁻ ions build up, forming alkaline NaOH solution.
Universal indicator turns bleached at the anode (due to Cl₂) and purple in the main part of the tube (due to NaOH).
How to decide which ion gets discharged:
• At the cathode: The ion lower in the reactivity series is discharged (e.g., H⁺ instead of Na⁺).
• At the anode: Halide ions are discharged if present. If not, OH⁻ is discharged.
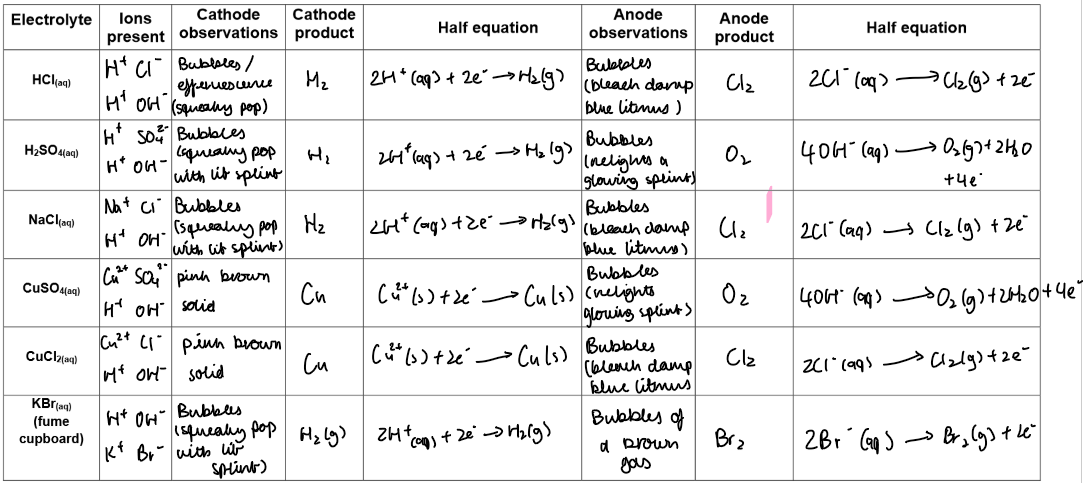
Complete the table:
Electrolyte | Ions present | Cathode product | Anode product |
|---|---|---|---|
HCl(aq) | H⁺, Cl⁻, OH⁻ | H₂(g) | Cl₂(g) |
H₂SO₄(aq) | H⁺, SO₄²⁻, OH⁻ | H₂(g) | O₂(g) |
NaCl(aq) | Na⁺, Cl⁻, H⁺, OH⁻ | H₂(g) | Cl₂(g) |
CuSO₄(aq) | Cu²⁺, SO₄²⁻, H⁺, OH⁻ | Cu(s) | O₂(g) |
CuCl₂(aq) | Cu²⁺, Cl⁻, H⁺, OH⁻ | Cu(s) | Cl₂(g) |
Electrolysis of aqueous solutions:
Electrolysis of dilute sulphuric acid using the Hofmann Voltameter:
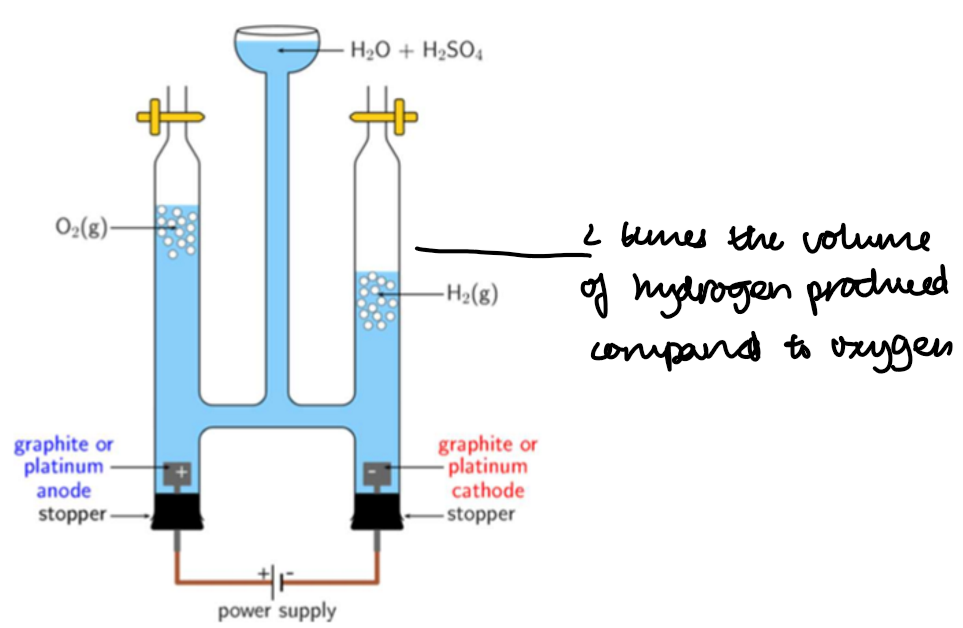
Observations and conclusions:
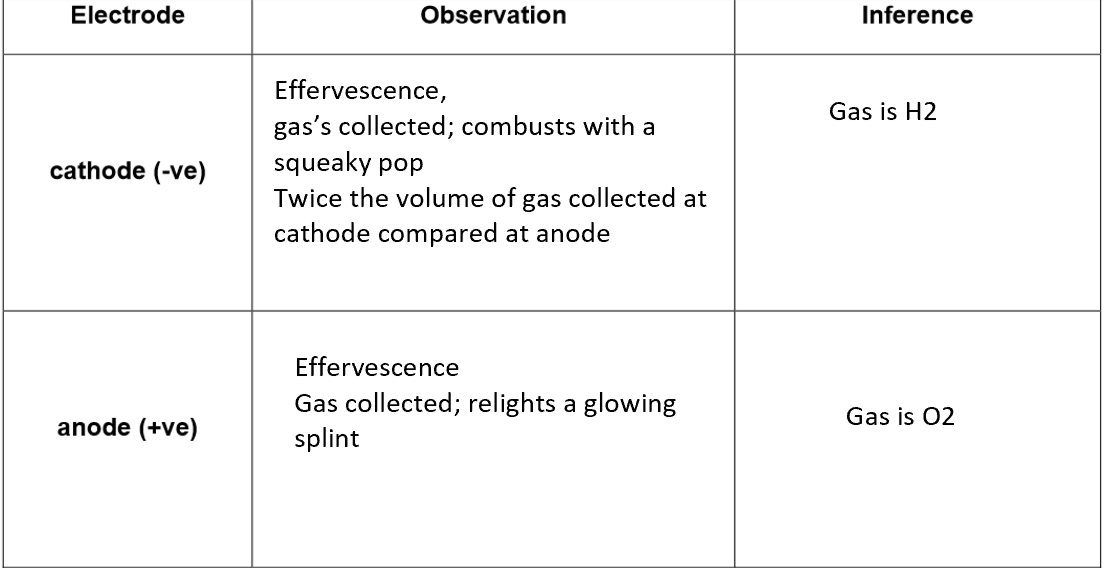
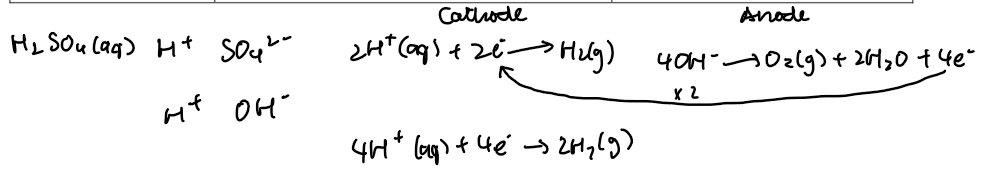
Conclusion
The ions in dilute sulphuric acid, H₂SO₄(aq), are H⁺, SO₄²⁻ and OH⁻.
At the cathode (–):
2H⁺(aq) + 2e⁻ → H₂(g)
• H⁺ cations (from acid and water) are attracted to the cathode.
• H⁺ ions are reduced to hydrogen gas.
• H⁺ ions are discharged.
At the anode (+):
4OH⁻(aq) → O₂(g) + 2H₂O(l) + 4e⁻
• OH⁻ anions (from water) are attracted to the anode.
• OH⁻ ions are oxidised to oxygen gas.
• OH⁻ ions are discharged instead of SO₄²⁻.
Why is twice as much hydrogen produced?
Because for every 4 electrons involved, 2 moles of H₂ form at the cathode, while only 1 mole of O₂ forms at the anode — due to the difference in the number of electrons needed per molecule (2 for H₂, 4 for O₂).