le chatelier's principle
<<march 30th, 2023<<
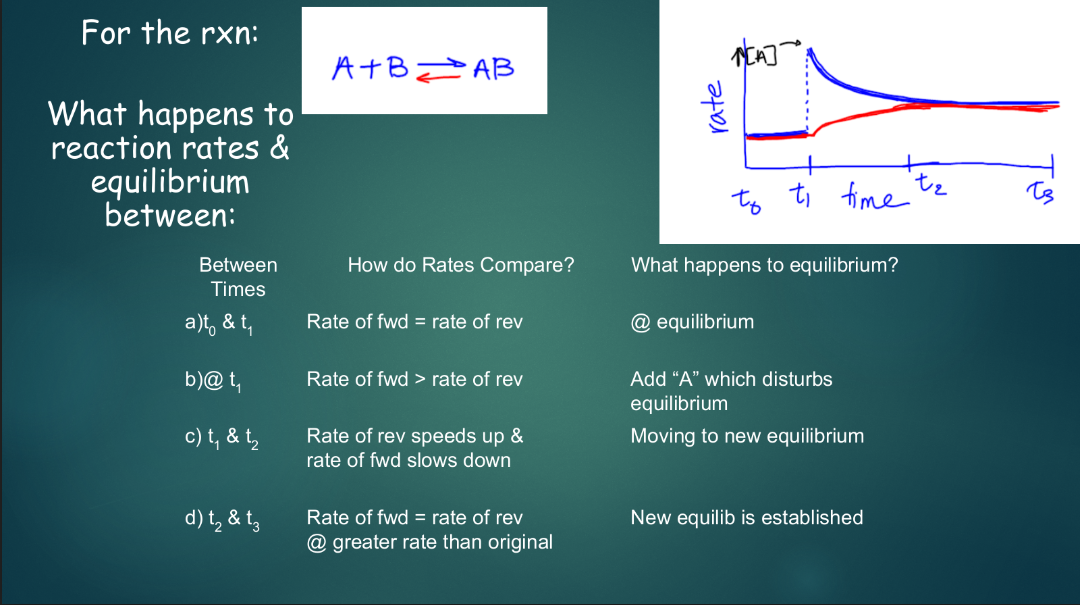
le chatelier’s principle: a law for how systems in equilibrium will behave when you change them
- if you change the concentration, temperature, volume, and/or pressure of a system, it will try to reach a new equilibrium
- we will focus on concentration, temperature, and pressure (for gases only)
you can use le chatelier’s principle by remembering ^^sss^^
- ^^system^^: some type of reversible process
- ^^stress^^: something that changes the system
- ^^shift^^: the system changes to establish a new equilibrium
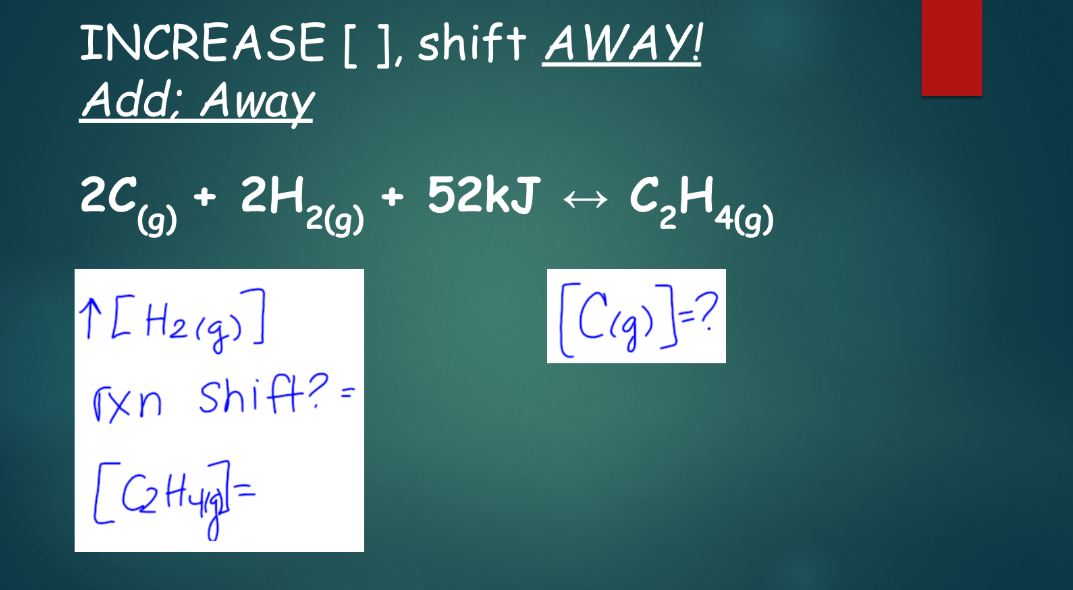
concentration in equilibrium:
think of this like a balance beam—both sides are equal, but what happens when you add or remove weight from one side? how do you balance that out?
- when more of a substance is added (concentration is increased), the equilibrium will shift away from the reaction that forms that substance
^^if more of a is added^^, the system will try to turn a into something else
^^if some of a substance is removed (concentration is decreased)^^, the equilibrium will shift toward the reaction that forms that substance
^^if a is taken out^^, the system will try to make more of a