Note
0.0(0)
Explore Top Notes Note
Note Studied by 22 people
Studied by 22 people Note
Note Studied by 101 people
Studied by 101 people Note
Note Studied by 28 people
Studied by 28 people Note
Note Studied by 10 people
Studied by 10 people Note
Note Studied by 30 people
Studied by 30 people Note
Note Studied by 141 people
Studied by 141 people
Ludi circenses: wagenrennen in het Circus Maximus
5.0(1)
9. Cellular Respiration
4.0(1)
Science Final Exam
5.0(1)
Chapter 49: An Introduction to Ecology
5.0(1)
APUSH: Period 3 Map Quiz
5.0(1)
Study Guide BFI - History Theme 1 : 1930-1945
5.0(1)
ionic bonding notes
Elements with low ionization energy want to lose valence electrons
elements that are electronegative want to gain valence electrons
-elements want to lose/gain electrons due to the octet rule
OCTET RULE- elements want 8 valence electrons to be stable
METHODS TO FIND IONS
Count electrons w/ lewis dot
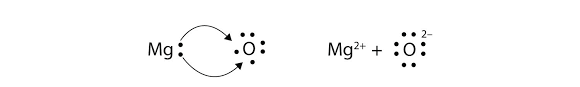
criss cross method
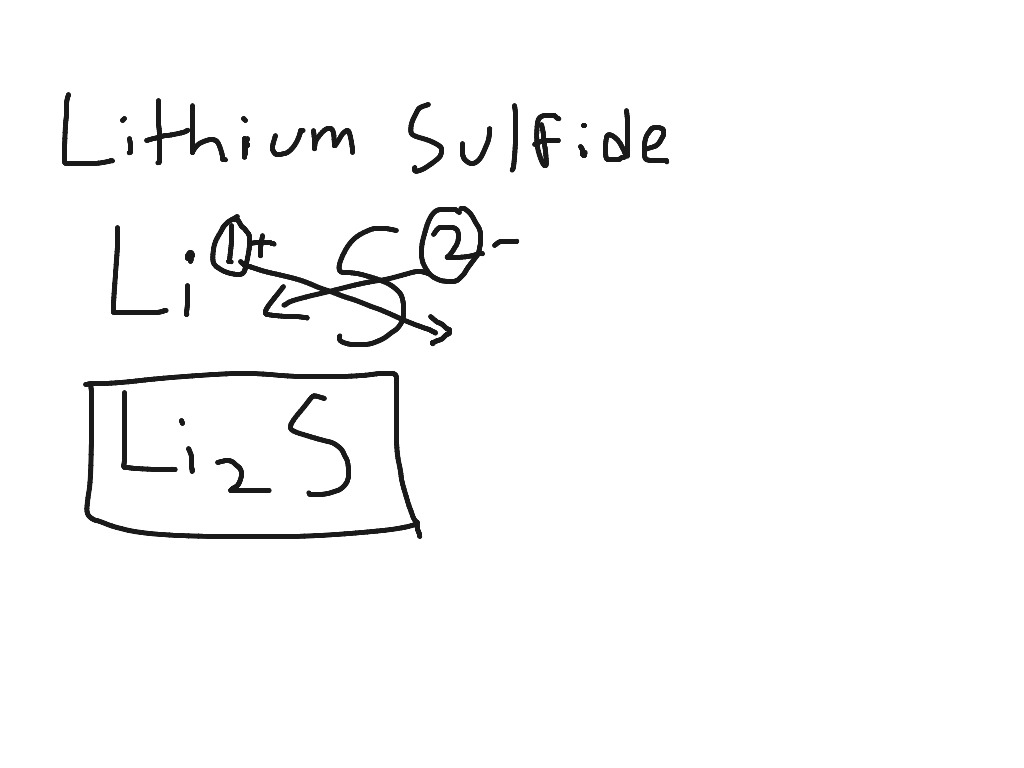
if each element has a charge of 1, you do not have to write the charges (EX: NaBr)
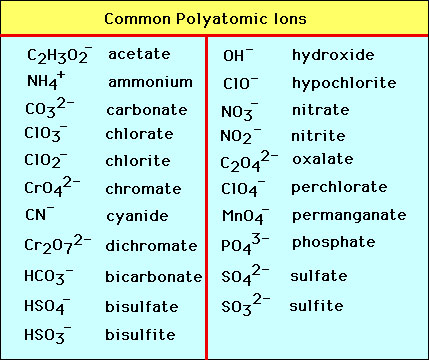
NAMING POLYATOMIC IONS
Cation (positive metal usually) comes first
Non metal comes second with “ide” at the end
THINGS TO KEEP IN MIND
if its a transition metal, you have to list the charge as a roman numeral (EX: Mn2 N3 → Manganese (II) Nitride)
if its a polyatomic ion, refer to the sheet
Note
0.0(0)
Explore Top Notes Note
Note Studied by 22 people
Studied by 22 people Note
Note Studied by 101 people
Studied by 101 people Note
Note Studied by 28 people
Studied by 28 people Note
Note Studied by 10 people
Studied by 10 people Note
Note Studied by 30 people
Studied by 30 people Note
Note Studied by 141 people
Studied by 141 people
Ludi circenses: wagenrennen in het Circus Maximus
5.0(1)
9. Cellular Respiration
4.0(1)
Science Final Exam
5.0(1)
Chapter 49: An Introduction to Ecology
5.0(1)
APUSH: Period 3 Map Quiz
5.0(1)
Study Guide BFI - History Theme 1 : 1930-1945
5.0(1)