Science Final Exam
Syllabus:
History of an atom
Structure of an atom
Bohr models
Organization of the periodic table
Lewis dot structure (ionic and covalent bonding)
Electronegativity
Physical and chemical change
Types of reactions
Balancing chemical equations
Photosynthesis
Cellular respiration
HISTORY OF AN ATOM
Democritus thought matter could not be created nor destroyed
Aristotle modified an earlier theory that matter was made of four “elements”: earth, fire, water, and air. He was incorrect.
John Dalton came up with several ideas including all matter is made up of atoms
JJ Thompson discovered the electron
Ernest Rutherford conducted the gold foil experiment, which led to the discovery of the atomic nucleus
Chadwick discovered the neutron, which further contributed to our understanding of atomic structure
STRUCTURE OF AN ATOM
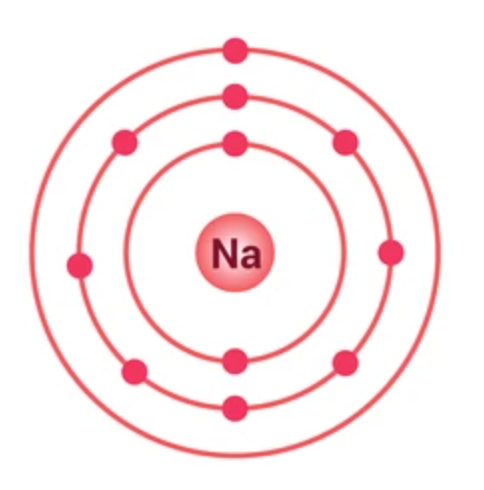
The atom consists of a central nucleus, made up of protons and neutrons, surrounded by a cloud of electrons that occupy specific energy levels
Protons have a positive charge, neutrons have a neutral charge, and electrons have a negative charge
2-8-18-32-32-18-8 (number of electrons on each shell)
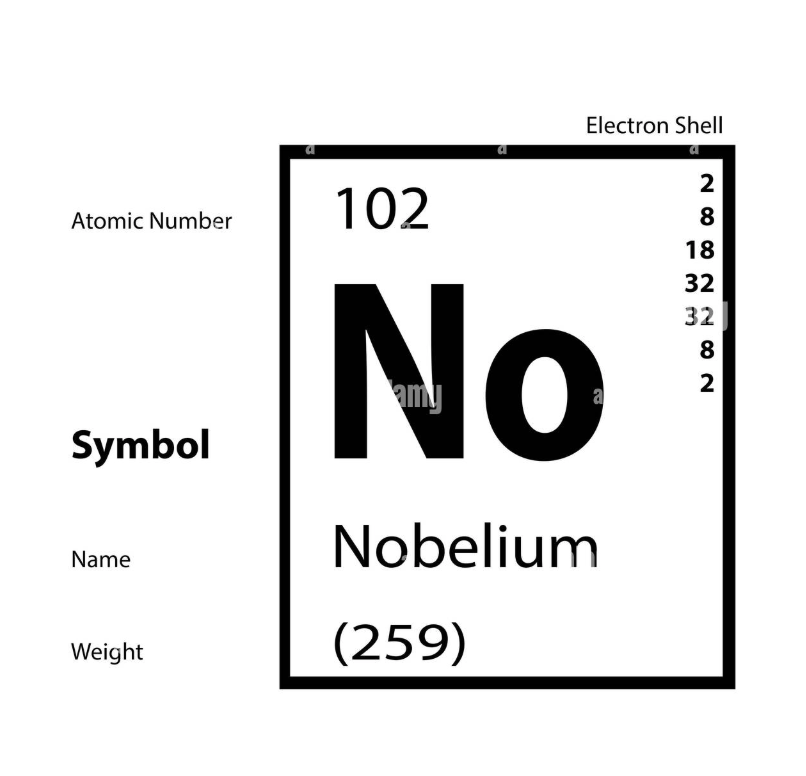
Atomic number: number of protons
Atomic number: number of electrons
Group: number of valence electrons
Period: number of shells
Mass number: number of protons and number of neutrons
An iscotope is when the protons and neutrons of an atom are not equal
BOHR MODEL
ORGANIZATION OF THE PERIODIC TABLE
Arranged in order of increasing atomic number
7 periods
18 groups
9 families
Alkali metals (shiny, soft, most reactive)
Alkaline earth metals (second most reactive family)
Transition metals (appear as metals, they are malleable and ductile and they conduct heat and electricity)
Post-transition metal (soft or brittle, with poor mechanical strength, and melting points lower than the transition metals)
Metalloids (properties of both metals and non-metals)
Other nonmetals (good insulators of heat and electricity)
Noble gases (rarely react with other elements)
Lanthanoids (produce a lot of energy when reacting with hydrogen, high boiling points and high melting points)
Actinoids (highly radioactive, unstable nucleus, metals tarnish in air)
Pnictogen, Chalcogens, Halogens (15, 16, 17 group on periodic table)
Boron Group or Earth Metals: Group 13 - three valence electrons
Carbon Group or Tetrels: Group 14 - four valence electrons
Nitrogen Group or Pnictogens: Group 15 - five valence electrons
Oxygen Group or Chalcogens: Group 16 - six valence electrons
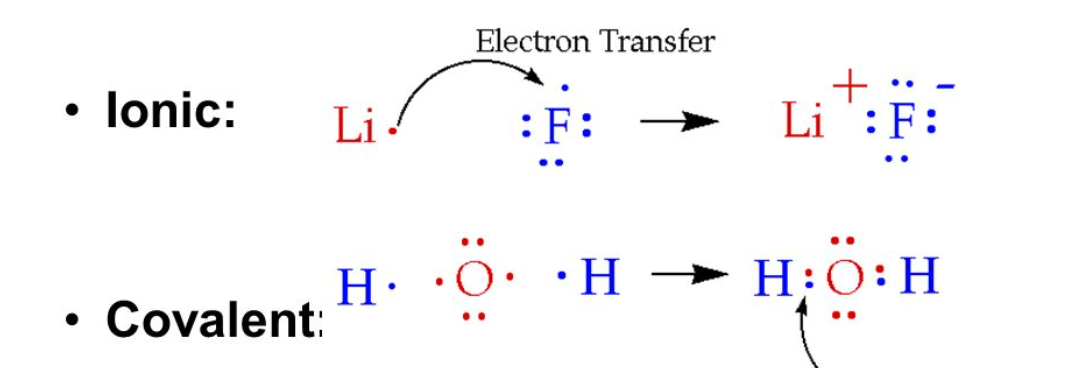
LEWIS DOT STRUCTURE
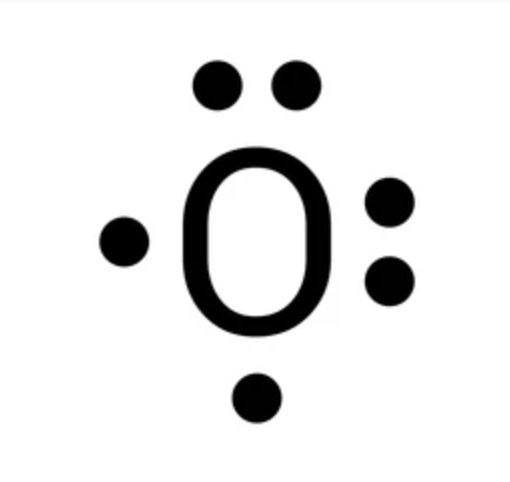
Covalent bond: sharing of electrons
nonmetal x nonmetal
Ionic bond: transfer of electrons
metal x nonmetal
ELECTRONEGATIVITY
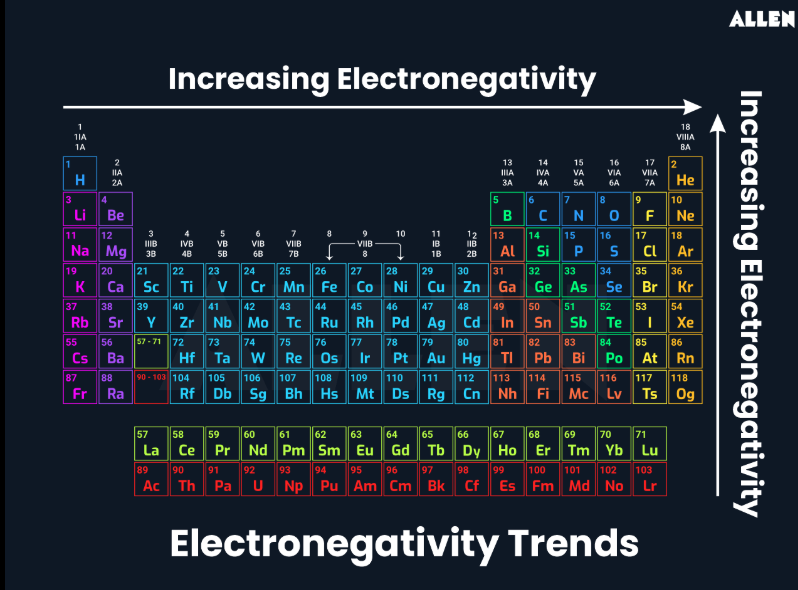
PHYSICAL AND CHEMICAL CHANGE
Physical change:
affect form but not chemical composition
reversible
no new substances are formed
a change in the matter identity
Chemical change:
one or more substances are formed
can’t be reversed
change in color (sometimes)
formation of gas
change in odor
change in temperature
produces light
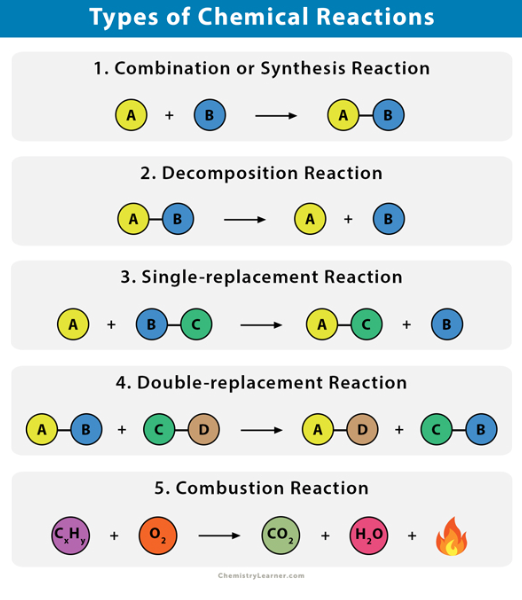
TYPES OF REACTIONS
Synthesis
Decomposition
Single-replacement
Double-replacement
Combustion
Endothermic: when energy is taken in from the surroundings
usually feels cold
Exothermic: when energy is transferred to the surroundings
usually feels hot
PHOTOSYNTHESIS
6CO2 + 6H2O → C6H12O6 + 6O2
Reactants: Carbon dioxide, water, energy from sunlight
Products: Glucose, oxygen
Most photosynthetic cells found in the leaves
Light-dependent reaction: 12 H2O + 12 NADP+ + 18 ADP + 18 Pi + light —> 6 O2 + 12 NADPH + 18 ATP
Calvin cycle: 3 CO2 + 6 NADPH + 5 H2O + 9 ATP —> G3P + 2 H+ + 6 NADP+ + 9 ADP + 8 Pi
Light-dependent reaction occurs in the chloroplast: thylakoid and stroma
Calvin cycle occurs in the stroma of the chloroplast
CELLULAR RESPIRATION
C6H12O6 + 6O2--> 6CO2 + 6H2O + ATP
Reactants: Glucose, oxygen
Products: Carbon dioxide, water, energy
Glycolysis: Glucose —> Pyruvic acid + 2 ATP
Krebs Cycle: Pyruvic acid —> CO2 + Chemical energy
Electron Transport System (ETS): O2 + Chemical energy —> H2O + 36 ATP
Glycolysis occurs in the mitochondria: matrix and cytoplasm
Krebs Cycle occurs in the matrix
ETS occurs in the cristae of mitochondria