Aleks Prerequisite Review | CHEM10401
Chemical Reactions
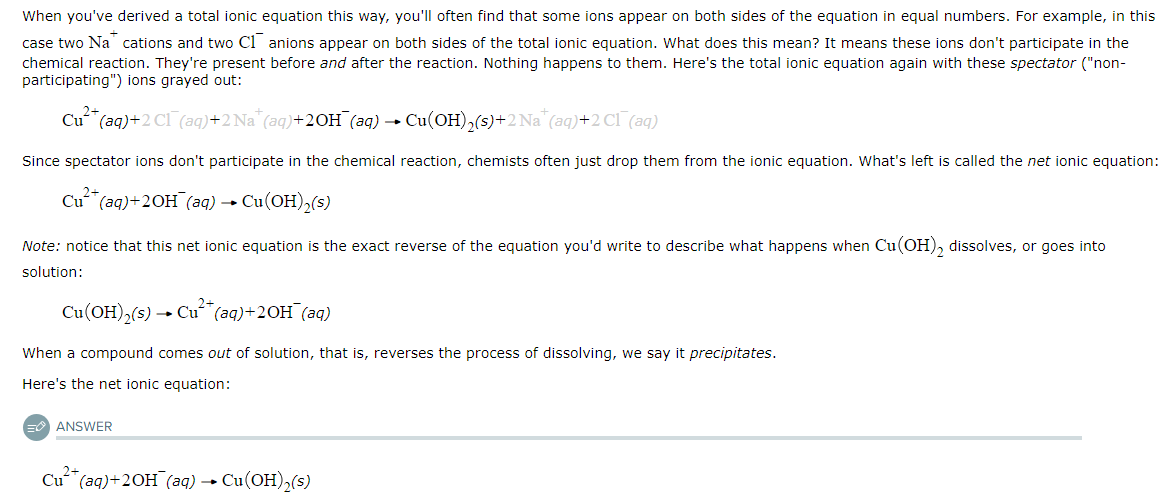
Writing Net Ionic Equations
The following chemical reaction takes place in aqueous solution:
CuCl2(aq)+2NaOH(aq)→CuOH2(s)+2NaCl(aq)
Write the net ionic equation for this reaction.

The following chemical reaction takes place in aqueous solution:
AgF (aq) + NH4Br (aq) → AgBr (s) + NH4F (aq)
Write the net ionic equation for this reaction.

The following chemical reaction takes place in aqueous solution:
AgNO3 (aq) + NH4I (aq) → AgI (s) + NH4NO3 (aq)
Write the net ionic equation for this reaction.

Thermochemistry
Calculating a molar heat of reaction from formation enthalpies
Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions:
2CH3OH (l) + 3O2 (g) → 2CO2 (g) + 4H2O (l)
Round your answer to the nearest kJ.

Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions:
2C2H6 (g) + 7O2 (g) → 4CO2 (g) + 6H2O (l)
Round your answer to the nearest kJ.
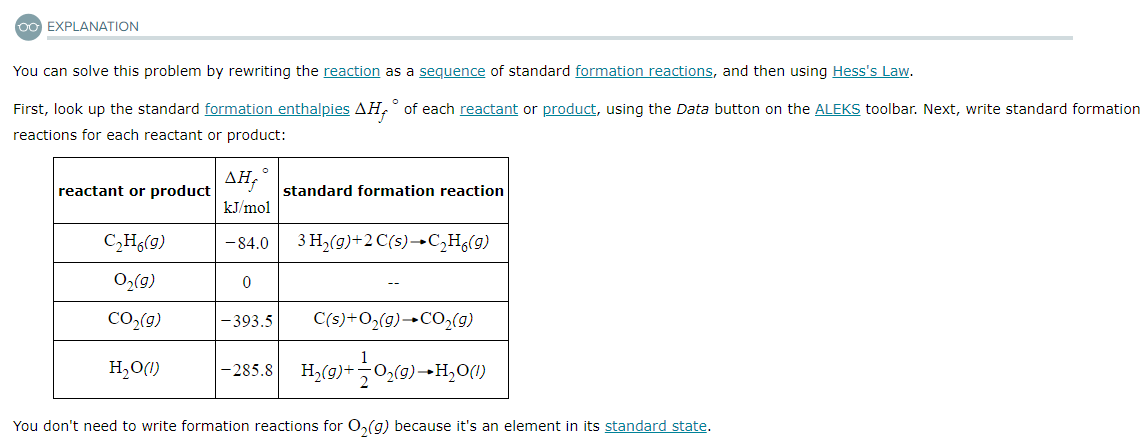
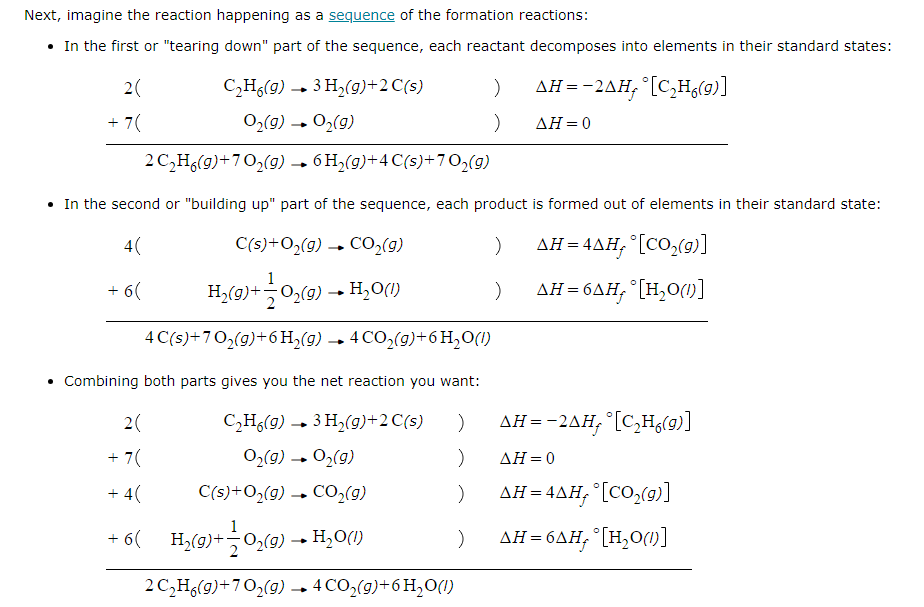
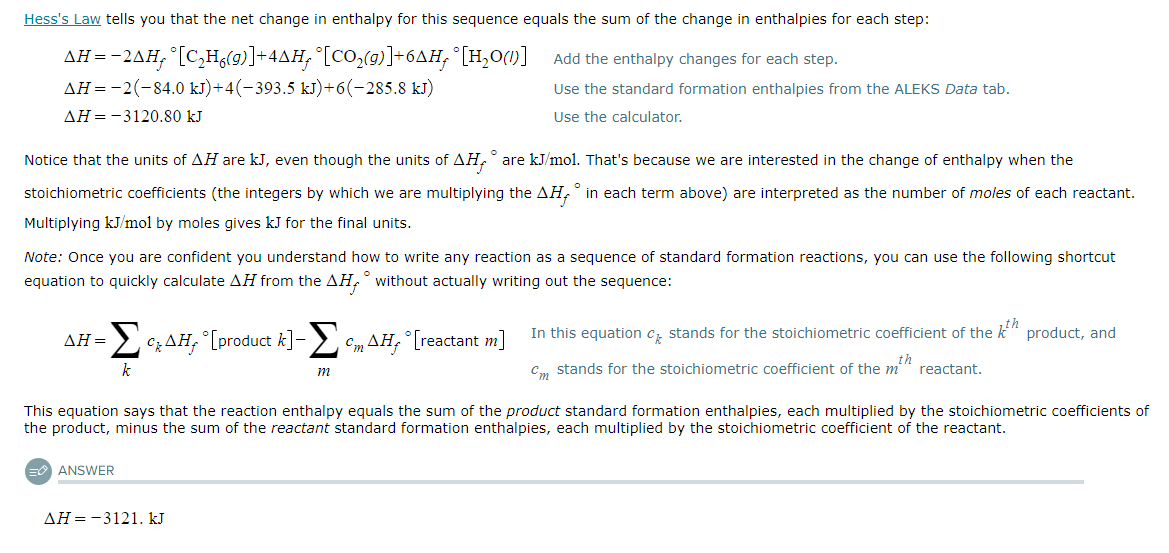
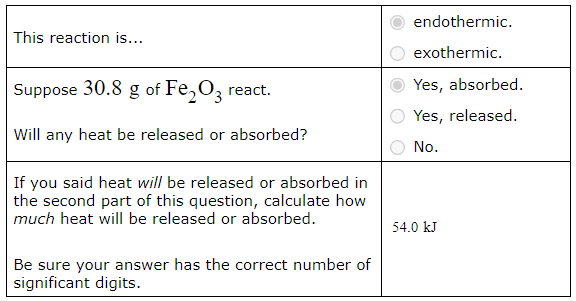
Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions:
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
Round your answer to the nearest kJ.

Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions:
2C6H6 (l) + 15O2 (g) → 12CO2 (g) + 6H2O (l)
Round your answer to the nearest kJ.

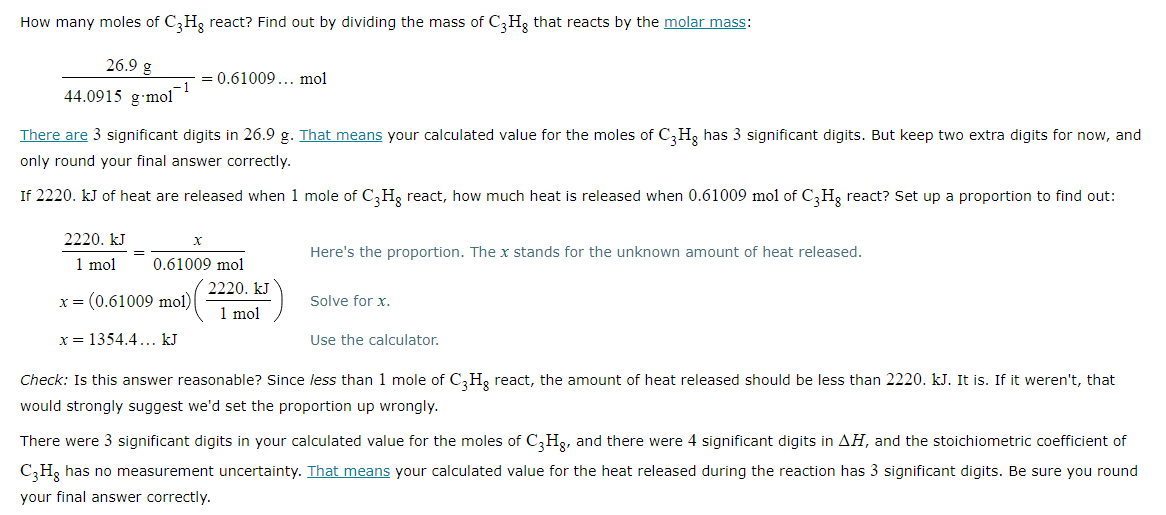
Calculating the heat of reaction from molar reaction enthalpy and the mass of a reactant
A chemist measures the energy change ΔH during the following reaction:
C3H8 (g) +5O2 (g) → 3CO2 (g) + 4H2O (l)
ΔH = −2220. kJ
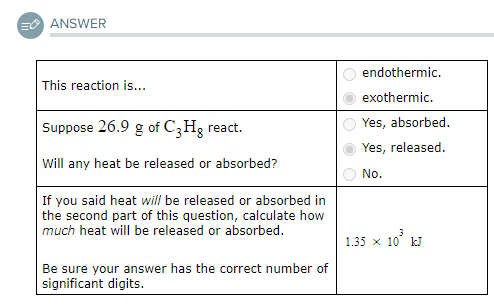
Use the information to answer the following questions.This reaction is...
endothermic.
exothermic.
Suppose 26.9g of C3H8 react.
Will any heat be released or absorbed?
Yes, absorbed.
Yes, released.
No.
If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed.
Be sure your answer has the correct number of significant digits.? kJ
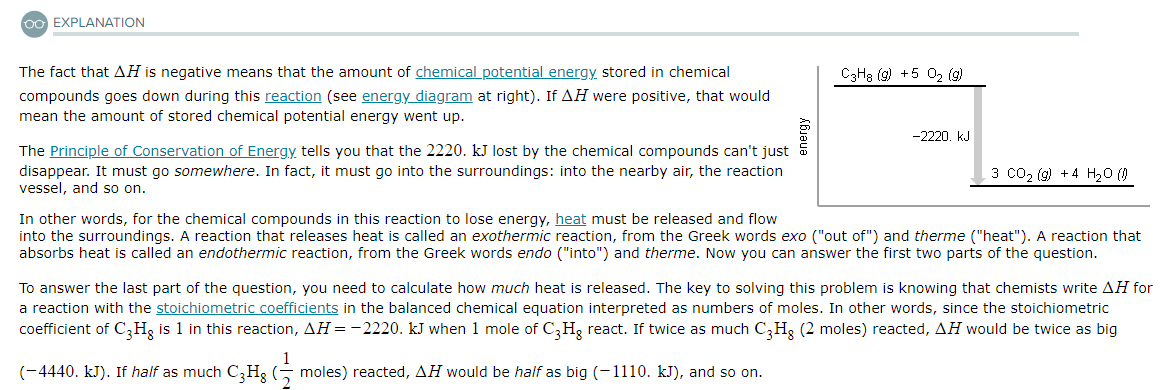
A chemist measures the energy change ΔH during the following reaction:
2H2O (l) → 2H2 (g) + O2 (g)
ΔH = 572. kJUse the information to answer the following questions.
This reaction is...
endothermic.
exothermic.
Suppose 84.1g of H2O react.
Will any heat be released or absorbed?
Yes, absorbed.
Yes, released.
No.
If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed.
Be sure your answer has the correct number of significant digits.? kJ

A chemist measures the energy change ΔH during the following reaction:
Cl2 (g) + H2 (g) → 2HCl (g) ΔH = −184. kJUse the information to answer the following questions.
This reaction is...
endothermic.
exothermic.
Suppose 93.8g of Cl2 react.
Will any heat be released or absorbed?
Yes, absorbed.
Yes, released.
No.
If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed.
Be sure your answer has the correct number of significant digits.? kJ
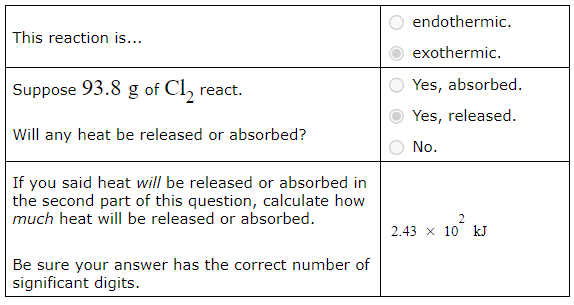
A chemist measures the energy change ΔH during the following reaction:
2Fe2O3 (s) → 4FeO (s) + O2 (g) ΔH = 560.kJUse the information to answer the following questions.
This reaction is...
endothermic.
exothermic.
Suppose 93.8g of Cl2 react.
Will any heat be released or absorbed?
Yes, absorbed.
Yes, released.
No.
If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed.
Be sure your answer has the correct number of significant digits.? kJ
States of Matter
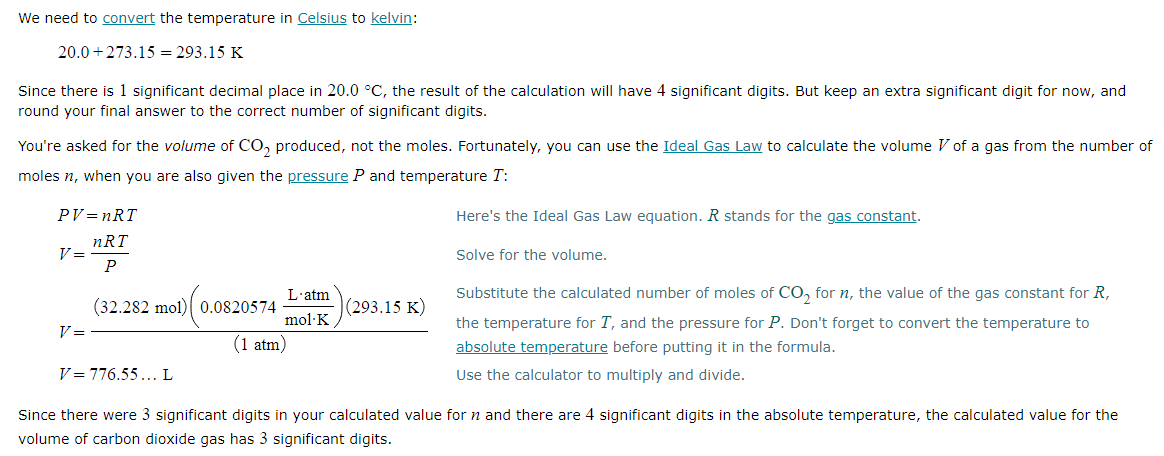
Solving for a gaseous reactant
Combustion of hydrocarbons such as nonane (C9H20) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.
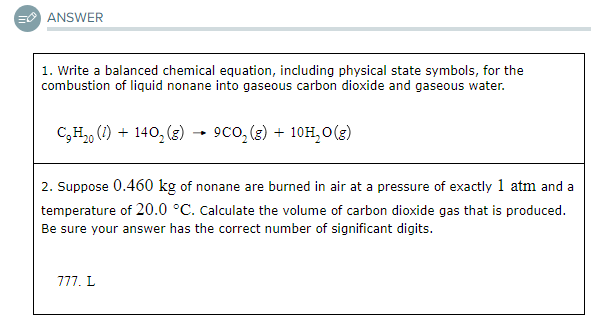
1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid nonane into gaseous carbon dioxide and gaseous water.
2. Suppose 0.460kg of nonane are burned in air at a pressure of exactly 1atm and a temperature of 20.0°C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits.
?L
Nitroglycerin is a dangerous powerful explosive that violently decomposes when it is shaken or dropped. The Swedish chemist Alfred Nobel (1833-1896) founded the Nobel Prizes with a fortune he made by inventing dynamite, a mixture of nitroglycerin and inert ingredients that was safe to handle.
Write a balanced chemical equation, including physical state symbols, for the decomposition of liquid nitroglycerin (C3H5NO33) into gaseous dinitrogen, gaseous dioxygen, gaseous water, and gaseous carbon dioxide.
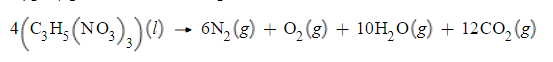
Suppose 16.0L of carbon dioxide gas are produced by this reaction, at a temperature of −8.0°C and pressure of exactly 1atm. Calculate the mass of nitroglycerin that must have reacted. Be sure your answer has the correct number of significant digits.
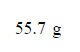
Combustion of hydrocarbons such as butane (C4H10) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason, there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.
1. Write a balanced chemical equation, including physical state symbols, for the combustion of gaseous butane into gaseous carbon dioxide and gaseous water.
2. Suppose 0.380kg of butane is burned in the air at a pressure of exactly 1atm and a temperature of 13.0°C. Calculate the volume of carbon dioxide gas that is produced. Be sure your answer has the correct number of significant digits.
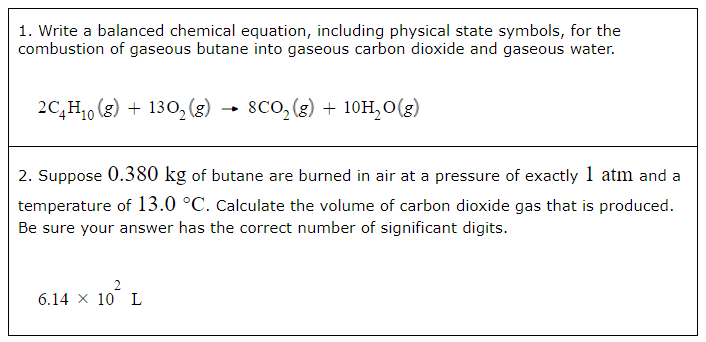
Ancient Romans built often out of bricks and mortar. A key ingredient in their mortar was quicklime (calcium oxide), which they produced by roasting limestone (calcium carbonate).
1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid calcium carbonate (CaCO3) into solid calcium oxide and gaseous carbon dioxide.
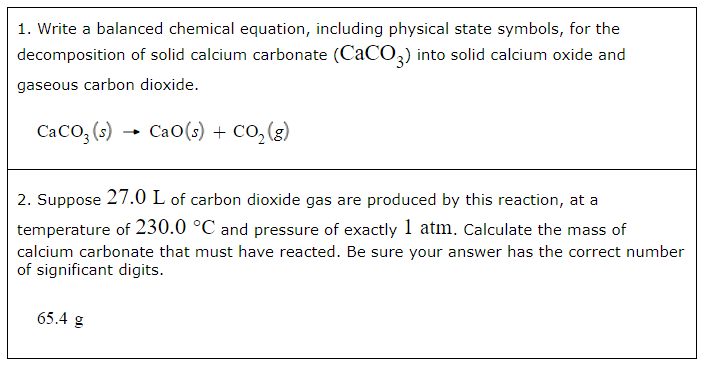
2. Suppose 27.0L of carbon dioxide gas is produced by this reaction, at a temperature of 230.0°C and pressure of exactly 1atm. Calculate the mass of calcium carbonate that must have reacted. Be sure your answer has the correct number of significant digits.
 Knowt
Knowt