bio topic 2
2.1 Molecules to metabolism
carbon atom is the core of organic compounds (exceptions i.e CO2 and CO)
C can form four covalent bonds, and thus allows for the formation of a wide variety of stable and complex compounds
some of these organic compounds essential for life i.e carbohydrates, proteins, lipids, nucleic acid
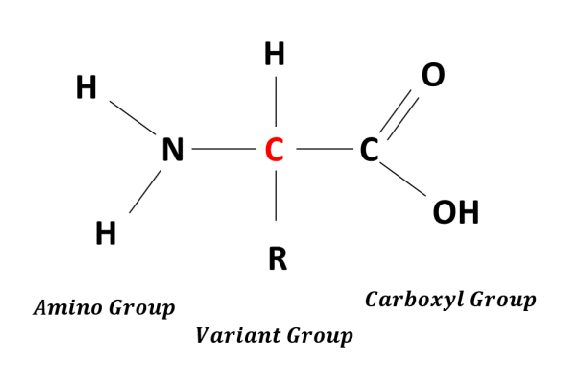
Amino acid
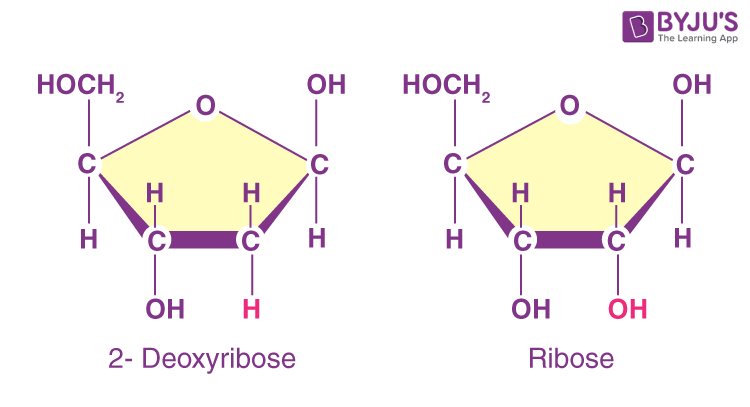
Ribose
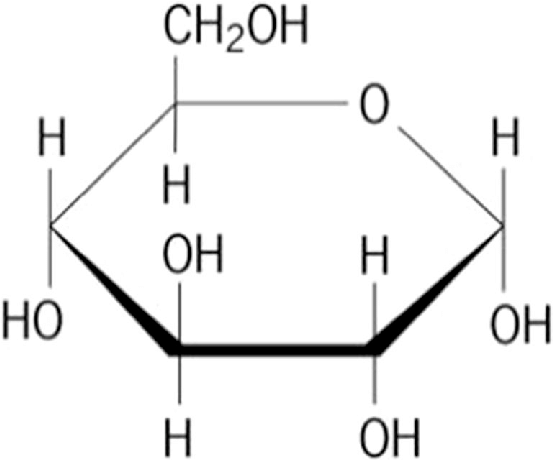
Glucose
Fatty acid
Skill: draw amino acid diagram
Skill: identify molecular diagrams of these structures as AAs, sugars (ribose and glucose) and lipids (fatty acids)
Metabolism
- web of all enzyme-catalysed reactions in a system (i.e cell or organism)
- metabolic pathways can consist of chains or cycles, can be anabolic or catabolic
Anabolism
- synthesis of complex molecules from simple ones, for example, formation of macromolecules from monomers by condensation reaction
- associated with condensation reactions, consist of removal of a water molecule each time a monomer is added a polymer chain or another monomer
- i.e: AAs → polypeptide + water
Catabolism
- breakdown of complex molecules into simpler ones, for examples, hydrolysis of macromolecules into monomers
- associated with hydrolysis, consists of addition of water molecules to break down a polymer
- i.e: dipeptide + water → 2 AAs
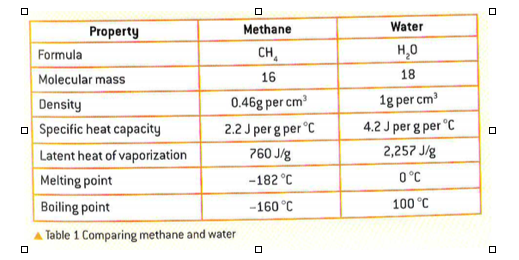
2.2 Water
- essential molecule for life on earth
- polar molecule
- consists of 2 H atoms bound by covalent bonds to an O atom
- principle of covalent bonding consists of sharing electrons between atoms
- polarity of water arises as it has slightly positively charged pole where the H atoms are located and a slightly negatively charged poled where O atom is located
Polar molecule = uneven distribution of charges across molecule
Non-polar molecule = even distribution of charges across the molecule, no positive or negative poles are formed
- watdue to polarity of water, small negative charges on O atom has ability to attract slightly positively charged H atoms in nearby H atoms from other molecules. this leads to formation of hydrogen bonds (covalent bond between atoms of SAME molecule, hydrogen bond between atoms of DIFFERENT molecule)
Thermal properties
- high specific heat capacity: large amount of energy needed to raise water’s temperature
- hydrogen bonds are said to be strongest of weak bonds as they restrict movement, takes lots of energy to break them down
- high latent heat of vaporization: H bonds between water molecules in a liquid form make it very hard for single molecules to escape as vapour
- energy necessary to break these H bonds and vaporize water is very high compared to other liquids (100 C)
- when water vaporizes, large release of energy occurs, causing cooling effect
- high latent heat of fusion: water at 0 C must lose a lot of energy before forming ice crystals, water expands as it freezes and therefore ice can float upon its surface
Cohesive properties
- water molecules can stick to each other through the formation of hydrogen bonds between the H of one and the O of another water molecule
- can explain formation of water droplets, why some organisms can “walk on water”, etc
Adhesive properties
- water can adhere to charged surface through the formation of H bonds due to its polarity
Solvent properties
- water is excellent solvent for other polar molecules that attract charged poles of water molecules (i.e inorganic molecules with +/- charges, polar organic molecules, enzymes etc)
- water can form bonds around other polar compounds i.e NaCl, separating them
- compounds and molecules that dissolve in water a referred to as hydrophilic
- water can also form H bonds around molecules whose elements are tightly bonded and thus act as an ideal transport medium for polar molecules (like glucose in blood)
Hydrophilic vs. hydrophobic substances
Hydrophilic: (“water-loving”) all molecules that can readily dissolve in water, include polar molecules and ionic compounds
Hydrophobic: (“water-hating) all molecules that cannot associate with water molecules or easily dissolve in it, include large and non-polar molecules, tend to be insoluble in water
• Glucose and amino acids are polar, so they can be freely transported and dissolved
in blood.
• Cholesterol and fats are non-polar so they are transported in small droplets called
lipoproteins, where these non-polar molecules are coated by phospholipids and
proteins, which are in turn, polar themselves.
• Oxygen is non-polar, and while some molecules can dissolve in water, they are not
sufficient to supply the entire body, therefore, most oxygen is transported in the
blood bound to hemoglobin.
2.3 Carbohydrates and lipids
Carbohydrates
- organic molecules composed of H, O, C atoms
- monosaccharides are the monomers (building blocks) of carbohydrates
- monosaccharides: glucose (G), fructose (F), galactose (Ga)
- disaccharides: maltose (G+G), sucrose (G+F), lactose (G+Ga)
- polysaccharides (all polymers of G): cellulose, glycogen, starch/amylose/amylopectin
Lipids
hydrophobic compounds that have important functions in:
long term energy storage
heat insulation
buoyancy
shock absorption
main monomers of lipids are fatty acids which may be:
saturated: all carbon atoms in fatty acid chain are connected by single covalent bond
monounsaturated: there is one double bond between two C atoms in fatty acid chain
polyunsaturated: more than one double bond between Cs in fatty acid chain
unsaturated fatty acids can be:
trans unsaturated: H atoms are bonded to carbon on the opposite sides of the double bond
cis unsaturated: H atoms are bonded to C on the same side of the double bond
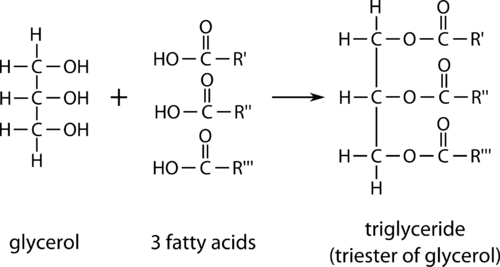
three main classes of lipids: phospholipids (important membrane components), steroids (cholesterol and hormones) and triglycerides (long-term energy storage)
Triglyceride formation
Both carbohydrates and lipids are suitable for energy storage:
| Carbohydrates | Lipids |
|---|---|
| more easily digested than lipids, good for energy storage that needs to be more rapidly releasedsoluble in water → easier to transport in blod | can store more energy per gram than carbohydrate → better for long term energy storagenot soluble in water, also harder to break down and transport around the body (build-up of high energy content fats) |
Health issues associated to trans and saturated fatty acids
- banned in several countries
- trans fats, saturated fats → coronary heart disease
BMI = mass in kg / (height in meters)^2
2.4 Proteins
Amino acids
contains carboxyl, ammine, an R group
monomers of proteins that when linked together by peptide bonds form complex proteins
proteins are important organic molecules that carry out major life functions in cells and in the extracellular space
20 different types of AAs, can be linked in any given sequence (proteins made out of n AAs, 20^n different proteins can be made)
specific sequence of each proteins is coded for in the genetic material of the organism
central dogma of molecular biology states that there is a sequential transfer of information where DNA is transcribed into RNA, which in turn is translated into proteins
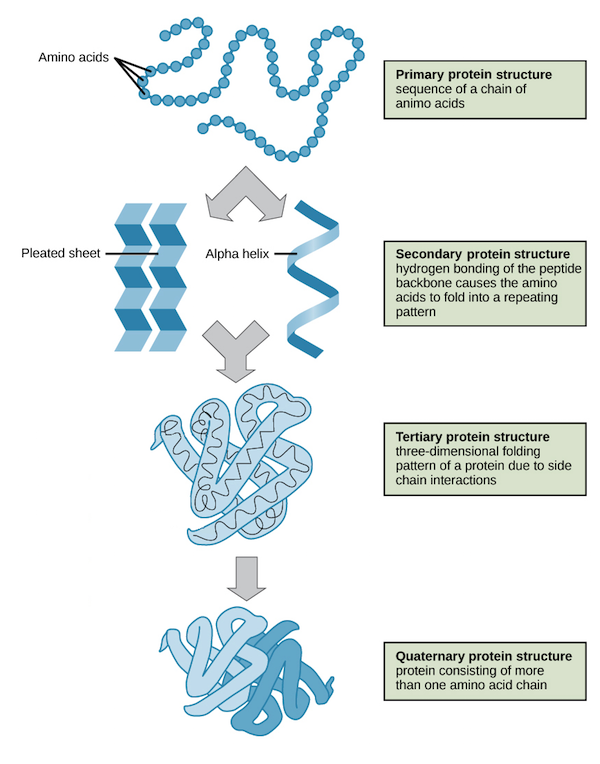
Protein structures
Peptide bond
- condensation reaction creates a covalent, peptide bond between carboxyl group of one AA and the amino group of the other, results in the release of water molecule
- in hydrolysis reaction water is added in order to break the petite bond
Function of proteins
| Function | Example | details |
|---|---|---|
| Structural transport | collagenhemoglobin | strengthen bone, tendon and skinbind oxygen in the lungs and transports to other tissues |
| Movement defence | actinimmunoglobulins | involved in the contraction of musclesacts as antibody |
Proteomes
- entire set of proteins expressed by a genome, cell, tissue, or organism
- each tissue or individual cell shows variable gene expression, thus different proteins are created
- proteome of individuals within the same species is quite similar (as the genetic make up is quite similar), however each individual has a unique proteome
2.5 Enzymes
- globular proteins that function as biological catalysts that speed up chemical reactions in the biological process
- substrates are substances acted upon by enzymes
- active site is the region on the enzyme to which substrates bind and where catalysis occurs
- activity of enzymes relies on the concepts of molecular motion and collision, substrates and enzymes must “collide” with other another due to their individual motion (kinetic energy), more collisions → faster reaction
- enzymes speed up reactions without getting consumed by the process, meaning they can speed up many reactions
- lock and key model: substrate and enzyme have shapes that make theme fit perfectly with each other; each enzyme catalyzes a specific reaction
- induced fit model: as substrate and enzyme approach each other, their interactions make them shift physical conformation so that they fit perfectly with one another
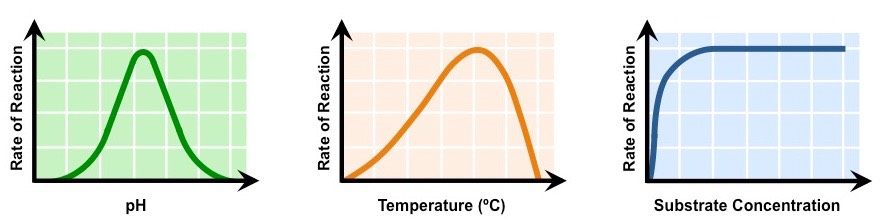
Influencing enzyme activity
Temperature
- enzyme activity increase as temperature increases, often doubling with every 10 C rise
- collisions between substrate and active site happen more frequently at higher temperatures due to faster molecular motions
- enzymes are proteins, therefore at high temperatures they are denatured and stop working, heat causes vibrations inside enzymes which break bonds needed to maintain the structure the structure of the enzyme
pH
- enzyme activity is reduced as pH increases above the optimum because the conformation of the enzyme is altered more and more
- above a certain pH the alkalinity denature the enzyme and it does not catalyze the reaction at all
Substrate concentration
at low substrate concentrations, enzyme activity increases steeply as substrate concentration increases
random collisions between substrate and active site happen more frequently with higher substrate concentrations
at high substrate concentrations most of the active sites are occupied, so raising the substrate concentration has little effect on enzyme activity
a plateau is reached when enzymes are working at full capacity at their maximum rate
Use of lactase in production of lactose-free milk
- many enzymes are used in industrial processes (i.e food industry)
- enzymes often immobilized on a surface and employed in large concentrations to catalyze a wide range of biochemical reactions
- lactose is the disaccharide in milk that many people are intolerant to as they do not produce the enzyme lactase to break it down
- often times milk and other milk products are treated with immobilized lactase, and lactose is broken down prior to consumption
- resulting monosaccharides are easier to digest by lactose-intolerant people, result in sweet flavor (less artificial additives needed)
- use of the enzyme also speeds up production of fermented products like yogurt and cheese
- immobilized lactase can be used in much larger concentrations and can resist larger changes in pH and temperature compared to endogenous (native) lactase
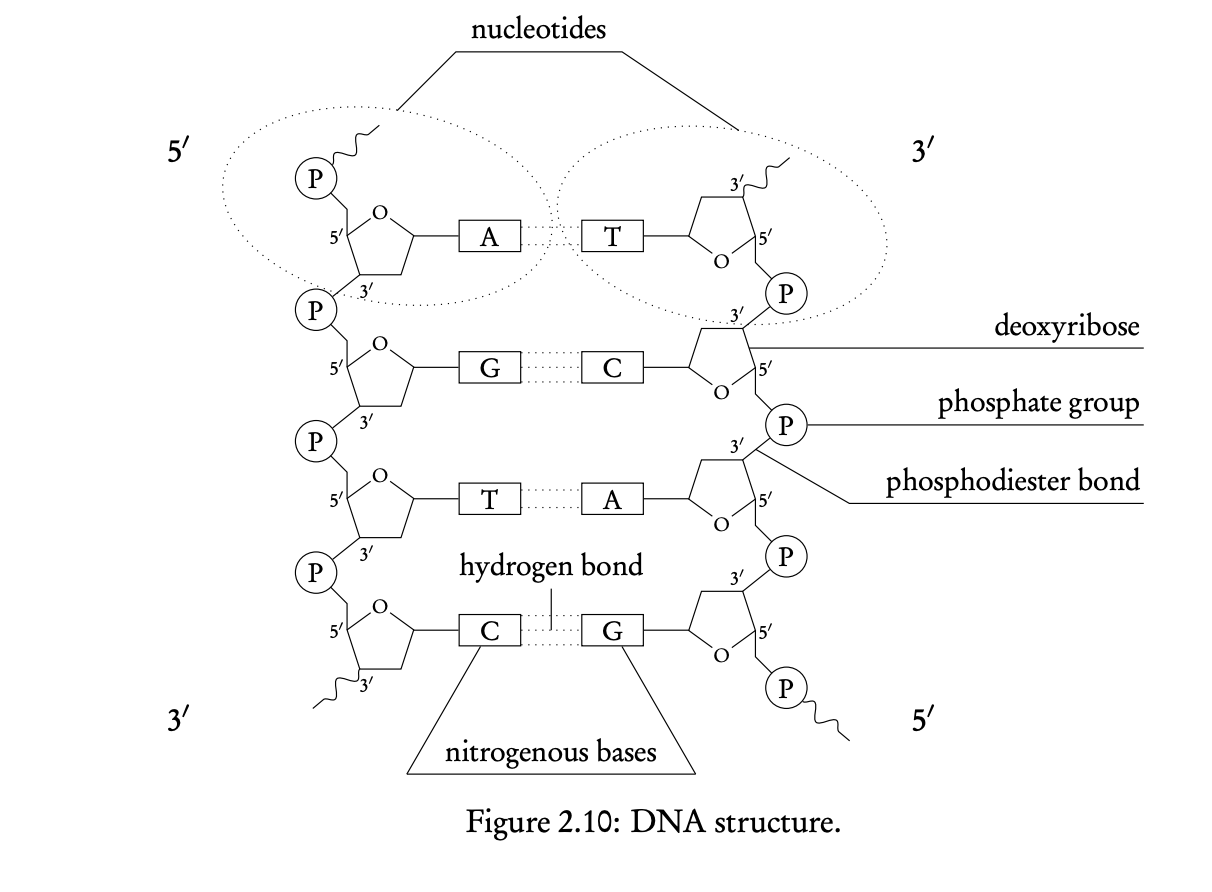
2.6 Structure of DNA and RNA
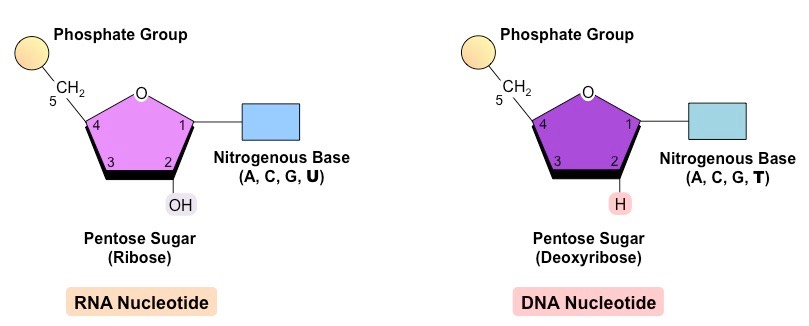
Nucleotide structure
nucleic acids are biomolecules responsible for information storage, essential to all forms of life
two major types of nucleic acids: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), essential compounds involved in gene expression in cells
RNA and DNA polymers consist of repeated units of nucleotides, which are made of a 5 carbon sugar linked to a phosphate group at carbon 5, and to one of five nitrogenous bases (adenine, guanine, thymine, uracil (ONLY RNA), cytosine)
nucleotide can have either a ribose (RNA) or a deoxyribose (DNA) pentose sugar
these differ in the presence of absence of an oxygen molecule, this oxygen molecule makes ribose a less stable molecule than deoxyribose, due to the fact that O has high electronegativity, meaning that it really wants more electrons
this instability causes RNA to be single stranded while DNA can be double stranded
Nucleotide structure
DNA vs. RNA
| RNA | DNA |
|---|---|
| contains a 5 carbon sugarsugar is called ribosesingle-stranded moleculecontains bases A, U, C, G | contains a 5 carbon sugarsugar is called deoxyribosedouble-stranded moleculescontains bases A, T, C, G |
Formation of DNA double helix
- DNA is composed of double stranded helix of DNA nucleotides
- each strand of DNA is held together by covalent bonds that form between the phosphate group of one nucleotide to carbon 3 of the neighboring nucleotide
- this forms a single-stranded backbone
- DNA double strand is then achieved by the formation of H bonds between the nitrogenous bases of two nucleotide strands
- base pairing in DNA is complementary, meaning that one base can only bind to a specific complementary base
- A binds to T → 2 H bonds
- C binds to G → 3 H bonds
- two DNA strands are antiparallel, in other words, they run in opposite directions (where on strand has a 5’ end, the complementary strands has a 3’ end)
2.7 DNA replication, transcription, and translation
DNA replication
- during the DNA replication process, one double stranded DNA molecules gives rise two daughter DNA molecules
- this process is said to be semi-conservative, meaning that each new DNA double helix constraints one newly synthesized daughter strand and one strand from the original DNA strand, which serves as a template to ensure that both new strands are identical (part of the original DNA is conserved at each replication step)
Brief description of process of DNA replication:
- takes place during the synthesis (S) phase of the cell cycle
- helicase unwinds the double helix and separates the two DNA strands for the new daughter strand to be synthesized
- enzyme DNA polymerase can then link free nucleotides to the template strands by the complementary base pairing. note that DNA polymerase can only add nucleotides at the 3’ end of a growing strand
- two identical daughter DNA strands are created, resulting in two semi-conservative double stranded DNA helices
Production of multiple copies of DNA by polymerase chain reaction (PCR)
- isolate the desired region of DNA (using restriction enzymes)
- introduce it in the a mixture containing free nucleotides, primers and Taq DNA polymerase
- the mixture is heated up to 90 C to separate the DNA strands of the original template
- temperature is then reduced to 55 C to allows for primer annealing to the now separated strands
- Taq polymerase (isolated from thermophiles, organisms that can survive at very high temperature) works optimally at 72 C so the mix is heated to this temperature to enhance the formation of new double-stranded copies of the original DNA
- process is repeated several times until the DNA is amplified
Transcription
- transcription is the synthesis of mRNA from the DNA base sequences present in an organism’s chromosomes
- the sections of DNA that code for polypeptides are called genes, but in order for these polypeptides to be expressed, machinery located outside the nucleus is needed
- thus a messenger RNA (mRNA) molecule carries the “message” from the DNA to the cytoplasm
- RNA polymerase unwinds the area of the DNA to be transcribed
- RNA polymerase catalyzes the addition of free RNA nucleotides using one of the newly separated DNA strands as a template for complementary base pairing (this creates a copy of the complementary DNA strand containing the gene of interest)
- in this process, thymine is replaced by nitrogenous base uracil (only present in RNA nucleotides)
- transcription occurs in a 5’ to 3’ direction
- once the whole gene has been transcribed, the resulting single-stranded mRNA molecules peels off and moves out of the nucleus to be translated into a polypeptide
Translation
- once the DNA “message” has entered the cytoplasm in the form of mRNA, translation takes place, where polypeptides are synthesized by ribosomes
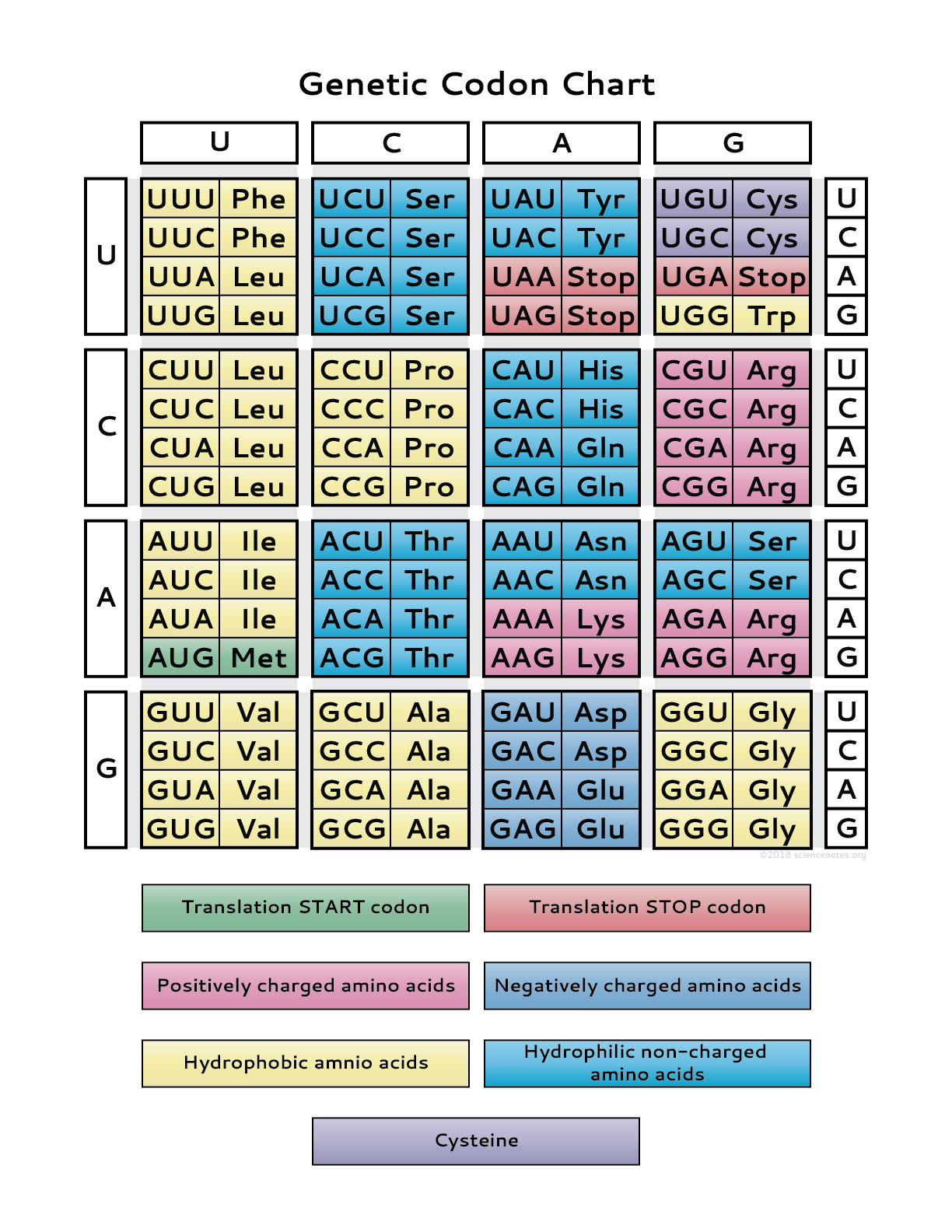
- genetic code is written in a language of codons (three consecutive bases (triple) where each codon codes for a specific AA)
- codons are located on the mRNA sequence, while anticodons (complementary codons) are found on tRNA molecules (type of RNA that carries the appropriate AA to the ribosome where translation occurs)
mRNA strand created during the process of transcription binds to a ribosome
ribosome begins to slide over the mRNA until it reaches a starting codon, where a tRNA with a complementary anticodon can bind, bringing the first AA of the polypeptide to be made
a second tRNA molecule with the appropriate anticodon binds to a second codon
ribosome catalyses the formation of a peptide bond between the two AAs, creating a dipeptide carried by the second tRNA
ribosomes slide over the mRNA molecule, leading to the release of the first tRNAs (the one that is no longer carrying an AA) and the binding of a new tRNA to the following codon
AA chain keeps growing as this process is repeated until a stop codon is reached, at which point the polypeptide breaks away from the tRNA and can fold and be modified to become a functional protein
Ribosome and tRNA
Producing human insulin in bacteria
- genetic code has been shown to be universal
- codon code is the same (one codon codes for the same AA in any organism)
- researchers have been able to synthesize important proteins at higher rates by introducing a human DNA sequence i.e in a smaller organism like E.coli, resulting in faster synthesis of desired proteins
- insulin is a great example of this; isolated insulin gene from humans and introduced it into E.coli (a bacterium that rapidly replicates and can yield large amounts of proteins in very short time periods), E.coli can then transcribe and translate the insulin gene using its innate machinery, can then isolate and purify this very important enzyme and use it for i.e treatment of diabetic patients
Skill: use a table of the genetic code to deduce which codon(s) corresponds to which AA. for example, try coding the following DNA sequence (only one strand is given) into its transcripted mRNA sequence and this sequence into separate AAs (remember to first find the start codon, and to correctly identify the stop codon, if present)
ACTACGTACCTGGGACTAGACT
UGAGCAUGGACCCUGAUCUGA
2.8 Cell respiration
Substrates and products
- cellular respiration is the controlled release of energy, in the form of ATP, from organic compounds in cells
- follows this equation: glucose + oxygen → carbon dioxide + water + ATP
- cell respiration can follow an aerobic (in the presence of O) and an anaerobic (no O) pathway, the latter creates much smaller yield of ATP
Anaerobic cell respiration
- no oxygen is available
- glycolysis occurs in the cell’s cytoplasm, where a glucose molecule is broken down into two smaller 3-carbon molecules called pyruvate
- this process leads to a smaller yield of ATP (2 molecules per reaction) and other products that can later be used in aerobic cell respiration
- in yeast cells, pyruvate is converted into ethanol and CO2 (there is not further yield of ATP and the products are released as waste), this process is known as fermentation
- in mammalian cells, pyruvate molecules are converted into lactate molecules (also known as lactic acid), with no further yield of ATP, lactate accumulate and can lead to changes in pH (lactic acidosis) which can be dangerous in the long-term)
Aerobic cell respiration
- oxygen is present, pyruvate can be further broken down in the cytoplasm and enter the mitochondria in the form of acetyl-CoA (a 2-carbon molecule)
- acetyl-CoA enters the Krebs cycle, where a series of redox reaction lead to the release of CO2 and the formation of intermediate molecules
- these molecules are used in the electron transport chain (at the mitochondrial membrane), resulting in a large yield of ATP (34-36 ATPs) and the release of water as a by-product
2.9 Photosynthesis
- process in which plants produce their own organic substances to be used as nutrients
- uses energy from the sun and simple organic compounds (water and CO2) to create complex carbohydrates to be used as fuel (mainly glucose) and oxygen:
- carbon dioxide + water → glucose + oxygen
Light spectrum and chlorophyll
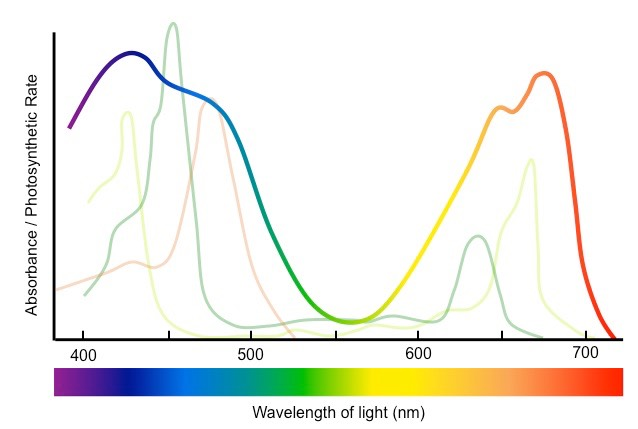
sunlight is made up of a range of wavelengths (red, green, blue) within the visible light spectrum
smaller the wavelength, more energy is reflected (blue)
larger the wavelength, less energy reflected (red)
green color is reflected from medium wavelengths
to absorb and reflect these light waves, specific pigments in plants needed
main photosynthetic pigment is chlorophyll, absorb red and blue light very well, reflects mostly green light (thus giving plants green color)
chlorophyll is located in clusters inside chloroplasts
Absorption + Action spectrum
Production of oxygen by photolysis
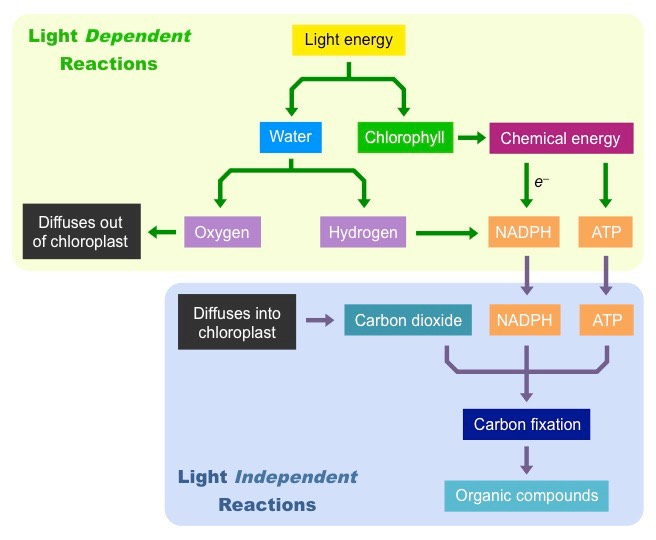
- photosynthesis consists of light-dependent and light-independent reactions
- light dependent reactions result in the yield of ATP, oxygen and hydrogen (light is absorbed by water, which is split to O and H)
Calvin cycle
- light-independent reactions lead to the formation of complex carbohydrates
- ATP and CO2 are used to convert inorganic compounds into organic compounds
- achieved by carbon fixation, which required energy from ATP
Rate-limitng factors of photosynthesis
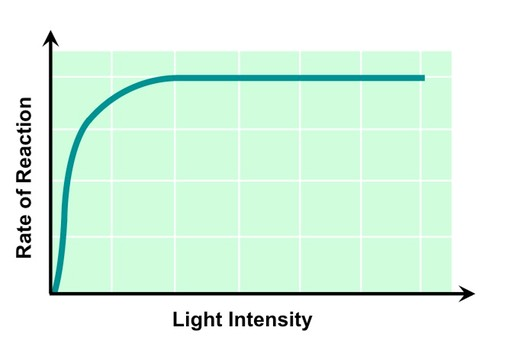
Light intensity
at low light intensities, rate of photosynthesis is limited
photolysis, which requires the absorption of light waves to slow down, so does O and ATP productions
indirectly limits the light-independent reactions, as ATP is necessary for carbon fixation to occur
graph levels off once all enzymes and reactions are occurring at highest speed possible
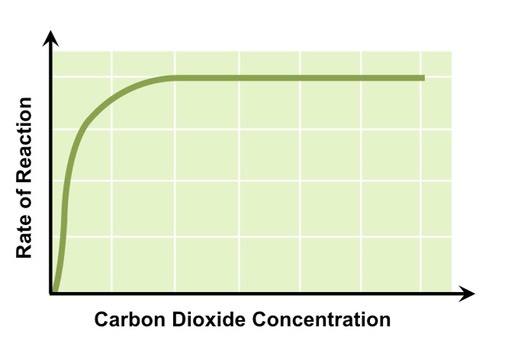
CO2 concentration
rate-limiting step in the Calvin cycle → carbon cannot be fixed to inorganic compounds and thus glucose production slows down
increasing CO2 concentration increases the rate of photosynthesis, until the photosynthetic enzymes involved in the cycle (i.e rubisco) reach their saturation point and can no longer increase rates
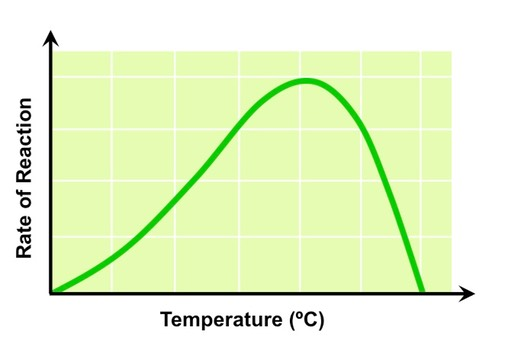
Temperature
- at low temperatures, the enzymes involved in photosynthetic reactions work very slowly
- rate of reaction increases steadily as temperature increases, until reaching an optimum point when all enzymes are working at a high rate
- when the temperature surpasses this optimal point, enzymes can be denatured, once again decreasing the photosynthetic rate
Skill: design experiments to investigate the effect of these factors on the photosynthetic rates
Photosynthesis overview