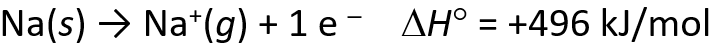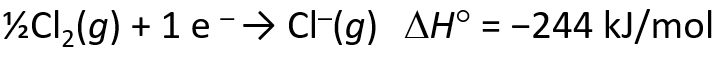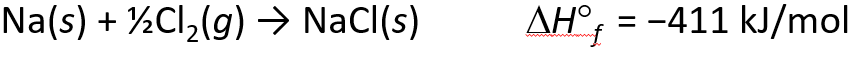Chem Knowt: Chapter 9
Chem Knowt: Chapter 9
|
|---|
- Potential Energy
- Due to position or composition
- Kinetic Energy
- Due to motion of the object
- Heat (q) is the transfer of thermal energy between two bodies at different temperature
- Heat flow (q) increases the thermal energy of one body and decreases the thermal energy of the other
- A change that releases heat is called an exothermic process
- A change that absorbs heat is an endothermic process
- Heat Capacity
- Heat Capacity (Cp): the quantity of heat needed to raise the temperature of some particular object by 1 degree C at constant pressure.
- q=Cp𝚫T
- Specific Heat (Cg): heat required to raise the temperature of 1 gram of a substance at 1 degree C at constant pressure
- q=mcs𝚫T
- Molar Heat Capacity (Cp): is the heat required to raise the temperature of 1 mole of a substance by 1 degree C at constant pressure
- q=ncp𝚫T
- Specific Heat of Water:
- Water can absorb a lot of heat energy without a large increase in its temperature due to its high specific heat capacity
- Heat Capacity (Cp): the quantity of heat needed to raise the temperature of some particular object by 1 degree C at constant pressure.
- System: the part of the universe that is the focus of a thermodynamic study. (Can be open, closed, or isolated).
- Surroundings: everything in the universe that is not part of the system
- Universe = system + surroundings
- First Law of Thermodynamics (The Law of Conservation of Energy)
- During a chemical or physical change, energy can be neither created nor destroyed although its form can change
- Work: is done when a force moves an object through a distance
- Lattice Energy: the extra stability that accompanies the formation of the crustal lattice is measured as the lattice energy.
- The Born-Haber Cycle is a hypothetical series of reactions that represents the formation of an ionic compound from its constituent elements
- Ion Size:
- The force of attraction between charged particles is inversely proportional to the distance between them
- Ion Charge:
- The force of attraction between oppositely charged particles is directly proportional to the product of the charges
Chapter 9: Thermochemistry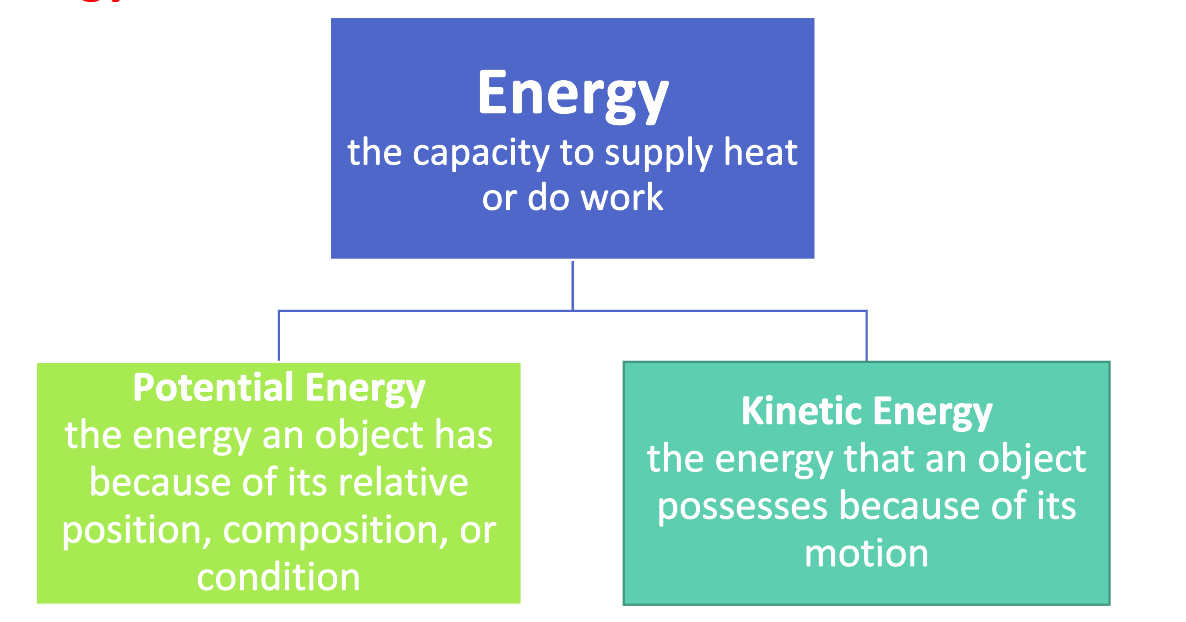
*Write Summaries for each chapter
Energy Basics
- Potential Energy
- Due to position or composition
- Chemical energy in a form of potential energy
- Potential Energy = mgh
- m = mass, g = force of gravity, and h = vertical distance
- Kinetic Energy
- Due to motion of the object
- Thermal energy is a form of kinetic energy
- Kinetic energy = ½ mv62
- m = mass, v = velocity
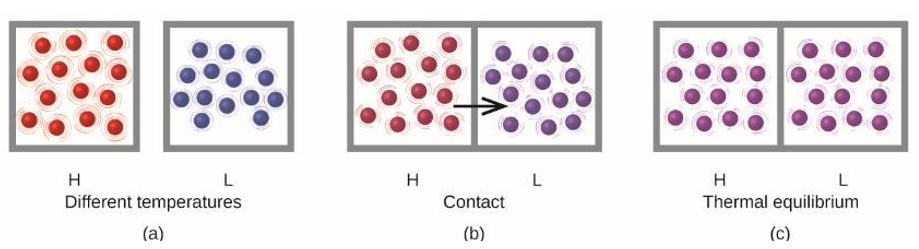
- Heat Flow
- Heat (q) is the transfer of thermal energy between two bodies at different temperature
- Heat flow (q) increases the thermal energy of one body and decreases the thermal energy of the other
- When two substances are placed in contact, thermal energy will always flow from the high temperature substance to the low temperature substance
- Heat flow will continue until both substances are at the same temperature
- Matter undergoing chemical & physical changes can release/absorb heat
- A change that releases heat is called an exothermic process
- Example: the combustion reaction that occurs in a campfire
- A change that absorbs heat is an endothermic process
- The reaction in a cold pack used to treat muscle strains
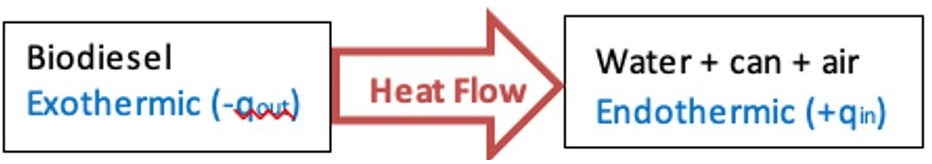
- Practice #1: Heat flow diagrams
- Example: Biodiesel burning below a can of water
- Energy Units
- Historically, energy was measured in units of calories (cal)
- Amount of energy required to raise one gram of water by 1 degree C
- The Calorie (capital C), or large calorie, commonly used in quantifying food energy content, is a kilocalorie
- The SI unit of heat, work, and energy is the joule
- Defined as the amount of energy used when a force of 1 newton moves an object 1 meter
- Named after James Prescott Joule
- 1 J = 1 kg m^2/s^2
- 1 calorie = 4.184 joules
- Historically, energy was measured in units of calories (cal)
- Heat Capacity
- Heat Capacity (Cp): the quantity of heat needed to raise the temperature of some particular object by 1 degree C at constant pressure.
- q=Cp𝚫T
- Specific Heat (Cg): heat required to raise the temperature of 1 gram of a substance at 1 degree C at constant pressure
- q=mcs𝚫T
- Molar Heat Capacity (Cp): is the heat required to raise the temperature of 1 mole of a substance by 1 degree C at constant pressure
- q=ncp𝚫T
- Specific Heat of Water:
- Water can absorb a lot of heat energy without a large increase in its temperature due to its high specific heat capacity
- The large amount of water absorbing heat from the air keeps beaches cool in the summer
- Without water, earth’s temperature would be about the same as the moon's temperature on the side that is facing the sun
- Water is commonly used as a coolant because it can absorb a lot of heat and remove it from the important mechanical parts to keep them from overheating
- Water can even prevent melting
- It can also be used to transfer the heat to something else because it is a fluid
- Heat Capacity (Cp): the quantity of heat needed to raise the temperature of some particular object by 1 degree C at constant pressure.
Calorimetry
- Terms Describing Energy Transfer
- System: the part of the universe that is the focus of a thermodynamic study. (Can be open, closed, or isolated).
- Surroundings: everything in the universe that is not part of the system
- Universe = system + surroundings
- An isolated system exchanges neither energy nor matter with the surroundings
- First Law of Thermodynamics (The Law of Conservation of Energy)
- During a chemical or physical change, energy can be neither created nor destroyed although its form can change
- Change in Internal Energy
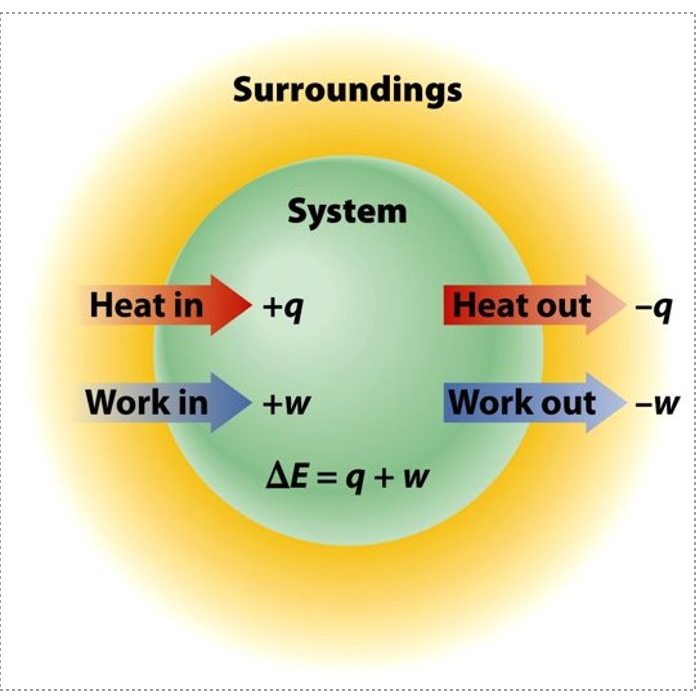
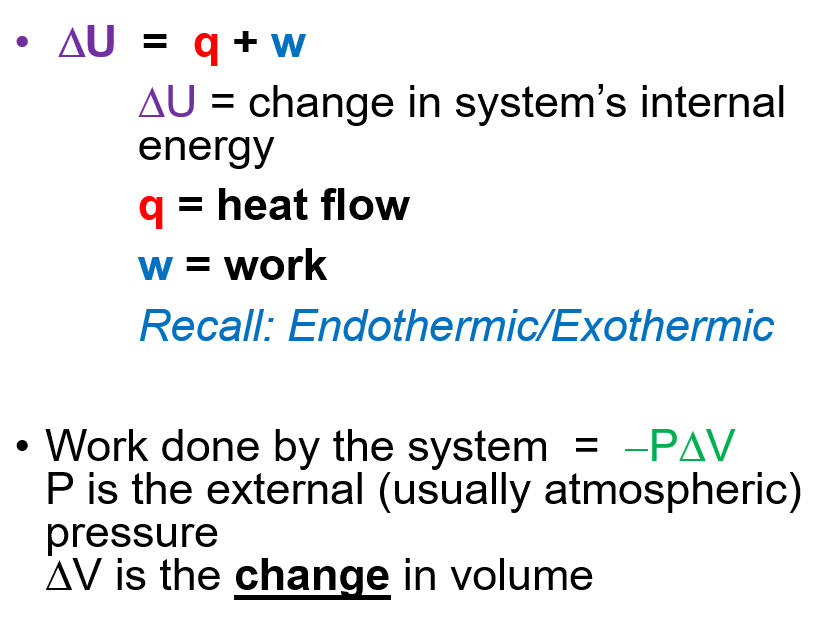
Enthalpy and Change in Enthalpy
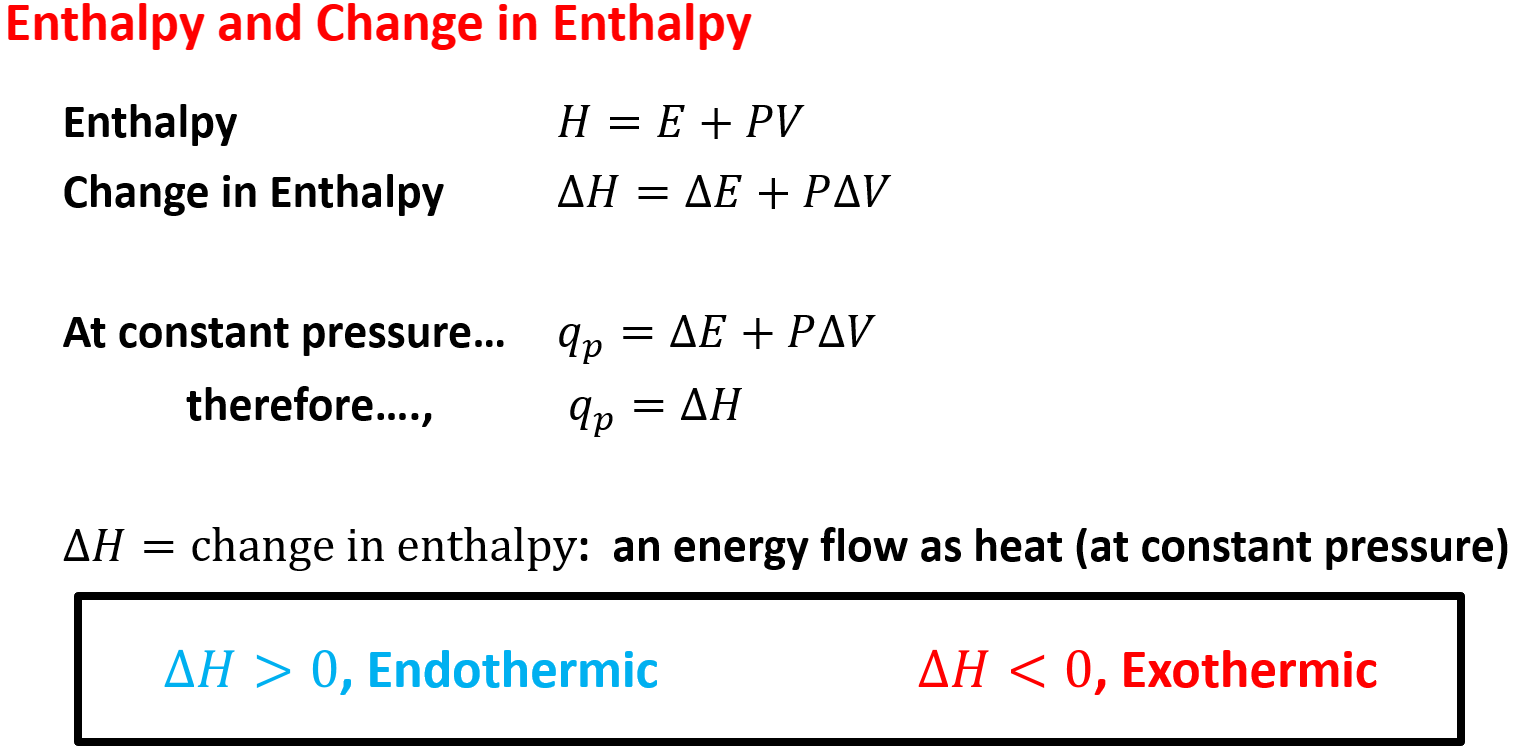
- Internal Energy (U) & Enthalpy (H) are… state functions!
- State functions only depend on the present state of the system, not how it arrived there
- State functions are independent of pathway
- State functions only depend on the present state of the system, not how it arrived there
- Work (W) & Heat Flow (q) are… non-state functions!
- Non-state functions dependent of pathway
- Work: is done when a force moves an object through a distance
- Heat Flow: is energy transferred between objects because of a difference in their temperatures.
- Heat of Reaction
- Or enthalpy of reaction, is the heat absorbed or released by a chemical reaction at constant pressure
- Methods of Determining Heat of Reaction
- From calorimetry experiments
- Calculations using others’ experiments:
- Using Hess’ Law
- From Enthalpies of Formation
- From Bond Energies
Method 1: Calorimetry
- The measurement of the heat flow that occurs during a physical change or chemical process
- A calorimeter is the device used to measure the flow of heat by a physical or chemical process
- Bomb Calorimeter: a constant-volume device used to measure the energy of a combustion reaction
Method 2: Hess’s Law
- The change in enthalpy for a stepwise process is the sum of the enthalpy changes of the steps
- If a reaction is reversed, 𝚫H sign changes
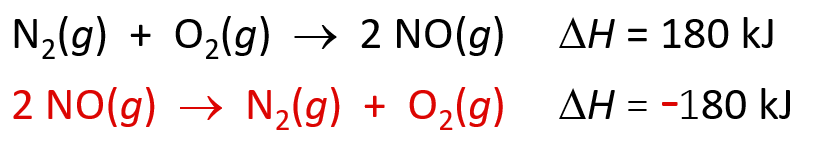
- If the coefficients of a reaction are multiplied by an integer, 𝚫H is multiplied by that same integer
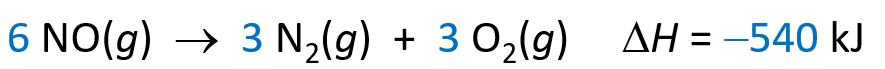
Method 2b: Enthalpies of Formation
- The standard enthalpy of formation (𝚫H f °) (standard heat of formations) is the enthalpy change of a formation reaction
- A formation reaction is the process of forming exactly one mole of a substance is in its standard state from its component elements in their standard states
- Practice: Write the standard enthalpy of formation reaction for nitric acid
- _____ → HNO3
- H2 + O2 + N2 → HNO3
- ½ H2 + 3/2 O2 + ½ N2 —> HNO3
- Formation of Oxygen Gas
- O2 (g) → O2 (g)
- 𝚫H f °Al (s) = 0
- Calculating 𝚫Hrxn degree from 𝚫Hf °values
- CH3OH(g) + _O2(g) → CO2(g) + _H2O(l) → 1CO2(g) + 2H2O(l)
- Use table 6.5: -238.6 kj/mol + 0 kj/mol → -395.5 kj/mol + 2(-285.8 kj.mol)
- 𝚫Hrxn = -726.5 KJ
- *practice problem
*Standard State = 0, look at Table 6.5
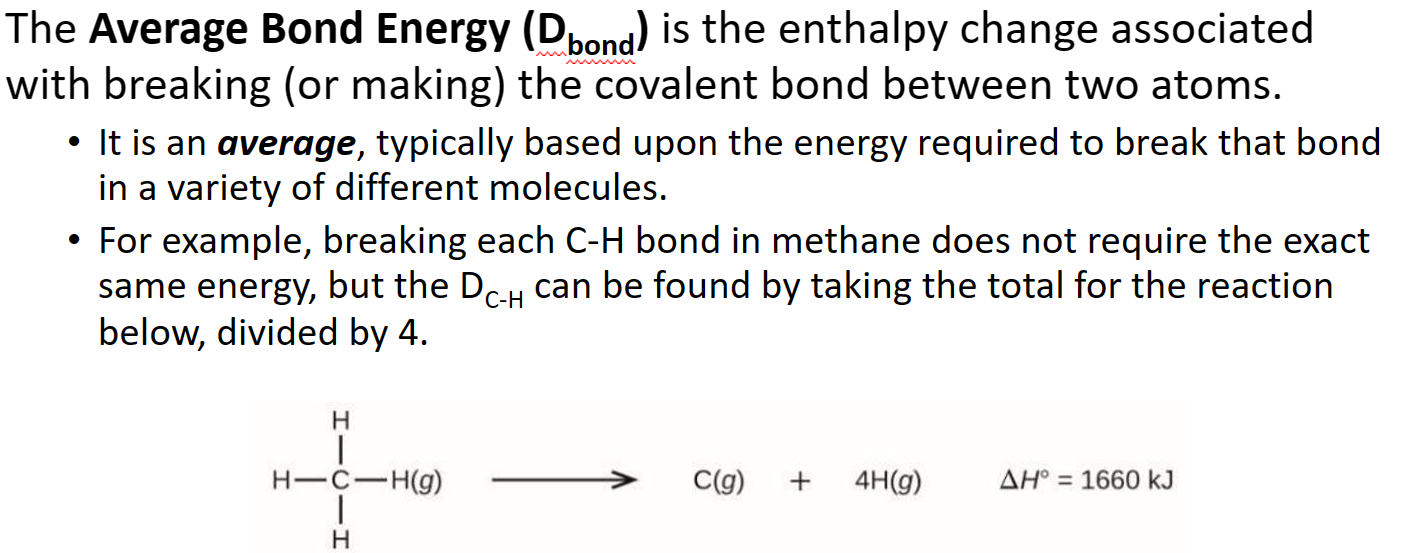
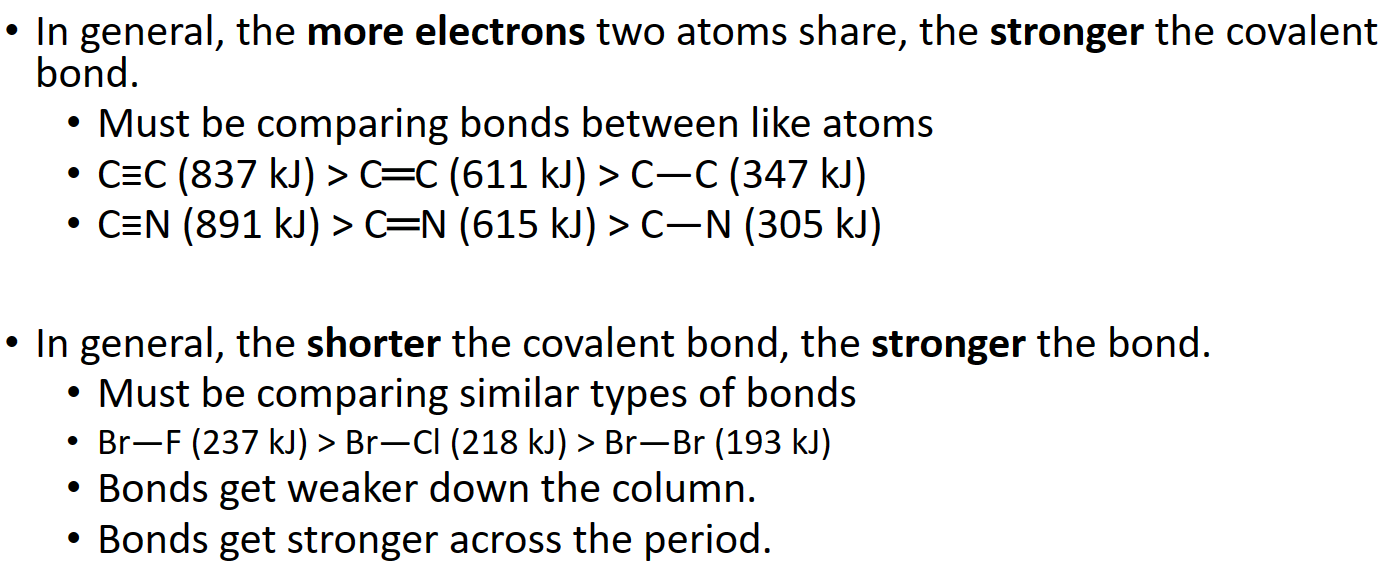
Method 2c: Bond Energies
 |  |
|---|
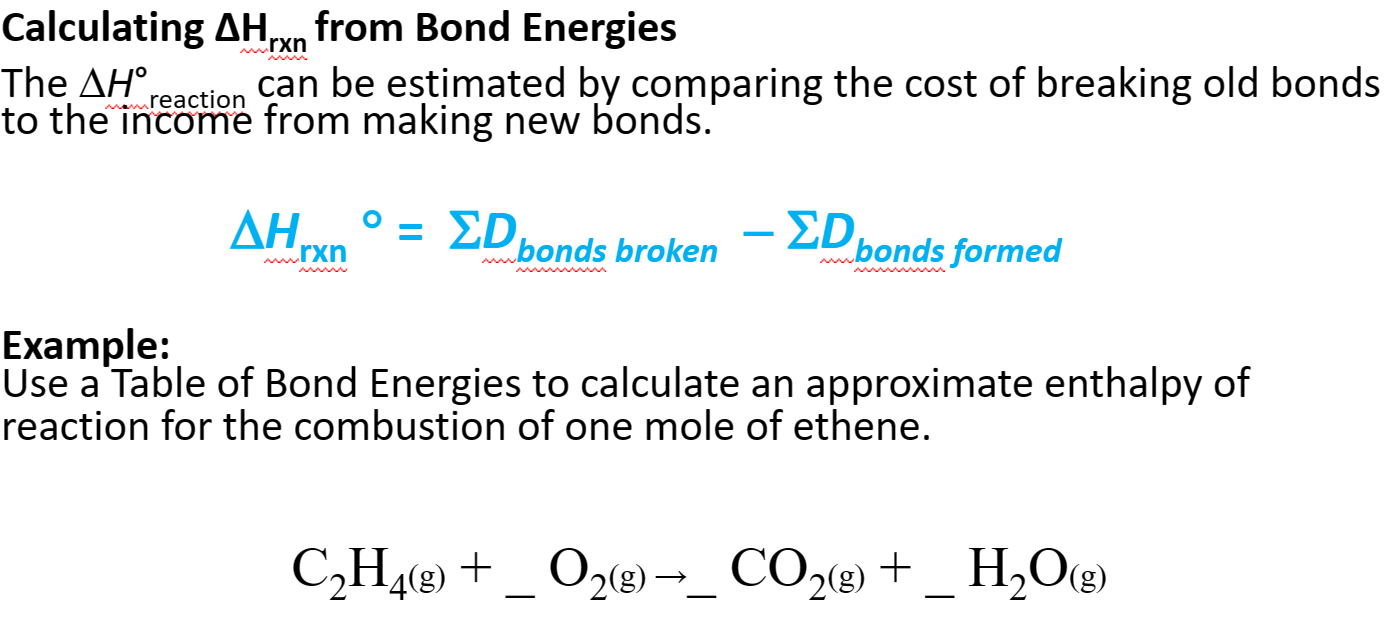
- Calculating 𝚫H rxn from Bond Energies
- The 𝚫H f °reaction can be estimated by comparing the cost of breaking old bonds to the income from making new bonds
Energetics of Ionic Bond Formation
- The ionization energy of the metal is endothermic
- The electron affinity of the nonmetal is exothermics
- But the heat of formation of most ionic compounds is exothermic and generally large. Why?
Ionic Bonding and the Crystal Lattice
- The extra energy that is released comes from the formation of a structure in which every cation is surrounded by anions, and vice versa
- The crystal lattice is held together by electrostatic attraction of the cations for all the surrounding anions
- The crystal lattice maximizes the attractions between cations and anions, leading to the most stable arrangement
- Lattice Energy: the extra stability that accompanies the formation of the crustal lattice is measured as the lattice energy.
- The lattice energy is the energy released when the solid crystal forms from separate ions in the gas state.
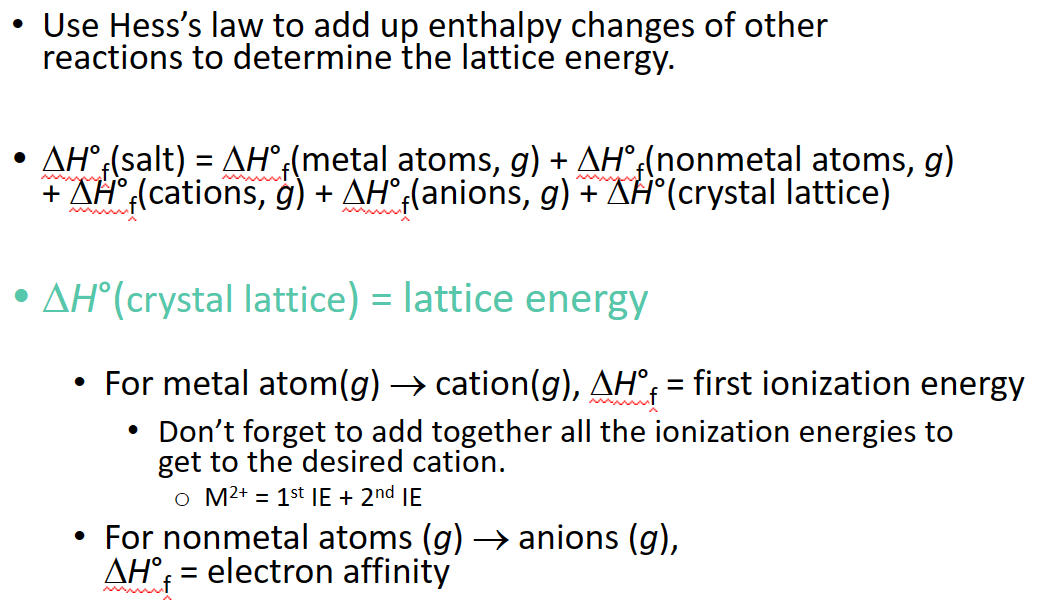
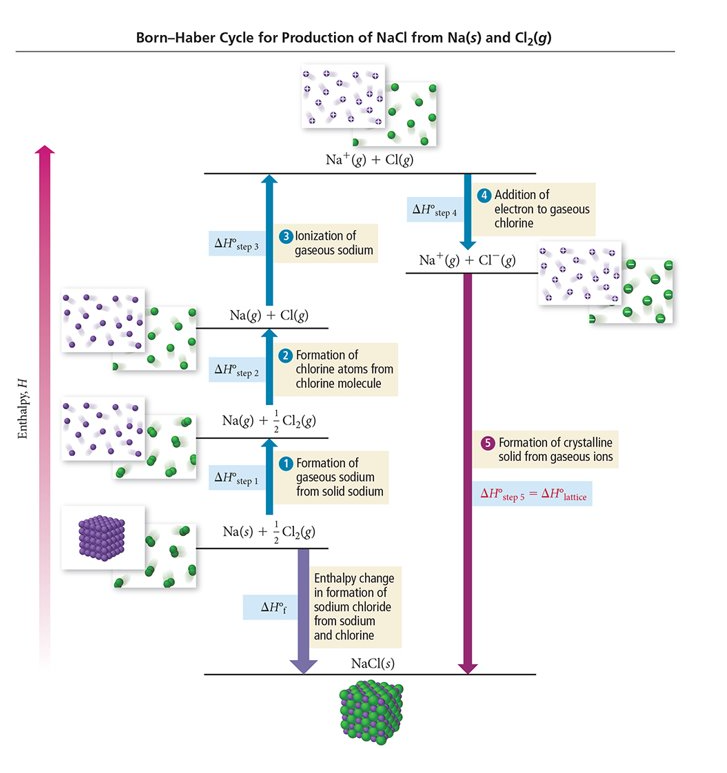
Determining Lattice Energy: The Born-Haber Cycle
- The Born-Haber Cycle is a hypothetical series of reactions that represents the formation of an ionic compound from its constituent elements
- The reactions are chosen so that the change in enthalpy of each reaction is known except for the last one
Born-Haber Cycle
 |  |
|---|
Trends in Lattice Energy
- Ion Size:
- The force of attraction between charged particles is inversely proportional to the distance between them
- Larger ions mean the center of positive charge (nucleus of the cation) is farther away from the negative charge (electrons of the anion)
- Larger ion: weaker attraction
- Weaker attraction = smaller lattice energy
- Ion Charge:
- The force of attraction between oppositely charged particles is directly proportional to the product of the charges
- Larger charge means the ions are more strongly attracted
- Larger charge = stronger attraction
- Stronger attraction = larger lattice energy
- Of the two factors, ion charge is generally more important