lecture 13: autophagy
overview and lysosomes
Autophagy was discovered by Ohsumi and is the removal of protein aggregates, old and damaged organelles and invading microbes as well as developmental remodelling. It also provides amino acids, nucleotides, lipids and sugars under low nutrient conditions.
A liver cell mitochondria has a life of about 10 days and old mitochondria are enclosed by a double membrane that creates an autophagosome to remove it through autophagy
Overview
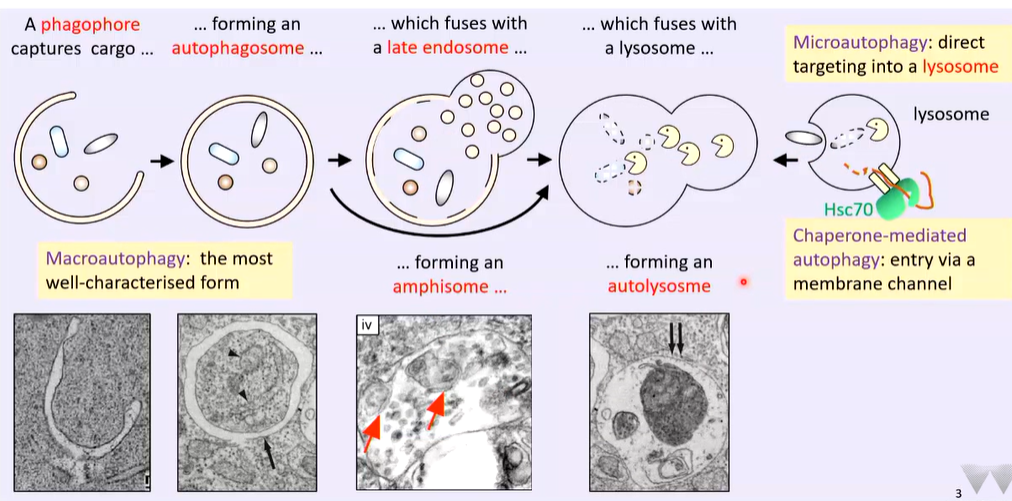
The end point is the lysosome which can engulf targets directly (microautotography), as well has having channels dedicated to chaperone mediated autophagy by HSC70 that directs a subset of proteins to the lysosomes through a channel where they are degraded.
Macroautophagy is the most characterized form. A phagophore forms and captures cargo that is not specifically targeted. The isolation membrane is associated with a tubular element and fuses to form an autophagosome. This fuses with a late endosome (characterized by vesicles in them) and forms an amphisome. The amphisome has small vesicles from the late endosome and a tubular and double membrane element. This fuses with a lysosome to form an autolysosome
Delivery of lysosomal enzymes (mammals)
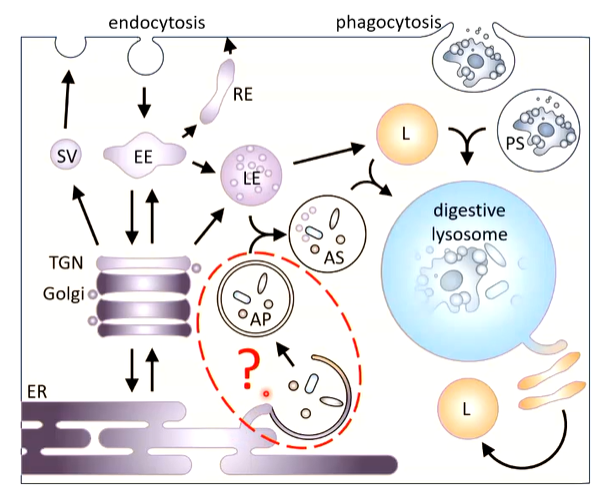
There is forward trafficked from the ER to the TGN which has multiple destinations such as the early endosomal system that has a pH of 6.5. Here it can go directly to the late endosome with a pH of 5.5. There is also backwards trafficking from the cell surface by endocytosis where the primary destination is the primary endosome that can access the late endosome and ER. To get to the lysosome you need lysosome late endosome fusion that forms an endolysosome.
There is ER associated expression of the protein directed into the protein that folds but is not activated. It is transported through the TGN and is still inactive. It is directed to the late endosome where there is a reduction in pH which starts to activate the protein. It is then directed t the late endosome where it is fully activated due to the low pH and is then delivered to the lysosome by fusion where it is fully activated. The endolysosome starts to tubulate which are cut off and fuse to form new lysosomes
These proteins are lipases, proteases, glycolipases, nucleases etc
lysosomal enzymes and maintenance of pH
The low pH is maintained by V type ATPases which convert energy derived from ATP hydrolysis into movement of h ions from the cytosol into the lumen of the lysosome. As H conc rises pH falls. This generates a transmembrane voltage so calcium ions are pumped out to that the pH can be regulated or the pumping in of chloride ions
Chaperone mediated autophagy and microautophagy
Chaperone mediated autophagy
There are two requirements - a dedicated protein translocation channel that is formed if it is a protein substrate that is formed by LAMP2. It has a transmembrane domain and a C terminate that sticks into the cytosol and is substrate binding. The N terminus has a domain that forms a channel
LAMP2A is focused on
It is also a dedicated channel for proteins carrying KFERQ motifs
This motif can be in any order and can be hard to spot. This a vague targeting motif and the overall features are required and not specific amino acids. This are always found by AI and are very common (40% of proteins have them)
In addition, it can be chemically modified so there are more active motifs:
This suggests that KFERQ driven SMA and microautophagy are used for the disposal of a wide range of substrates.

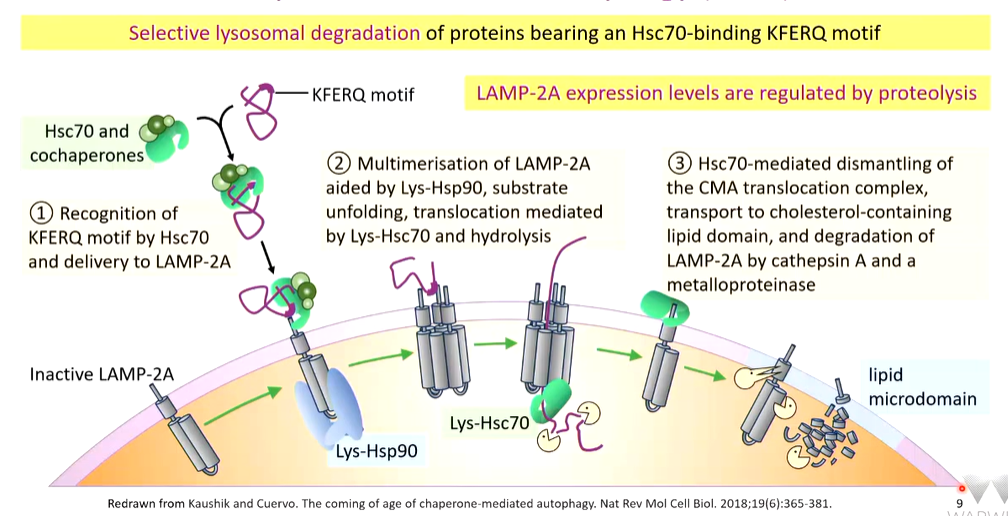
Chaperone mediated autophagy (CMA) overview
a KFERQ motif bearing substrate that has to be cleared is captured by HSC70 or specialised co-chaperones. These are delivered to a LAMP2A monosomer. Multimerization of LAMP2A aided by Lys-Hsp90, substrate unfolding, translocation mediated by Lys-HSC70 and hydrolysis. Once it is in the lumen, the protein is degraded. If there are excess LAMP2A, they are captured by HSC70 and directed to lipid microdomains that have high cholesterol concentrations and ae the site of proteolysis of LAMP2A by cathepsin A and metalloproteinases.
Down regulation
It can be down regulated by having a nutrient rich or high fat diet or age. This stimulate the activity of mTORC2 that inhibits CMA. It has a kinase activity that phosphorylates Akt1 that phosphorylates GFAP. GFAB keeps LAMP2A inactive and inhibits CMA.
up regulation
If food is withdrawn or growth factors and withdrawn or a range of cell stressors are supplied, CMA is upregulated. This is because they inhibit mTORC2. There is also pharmacological intervention that does this as well. This reduces the activation of AKT1 and GFAP. GFAP is required for the stabilization of the trimeric channel
Links with health and disease
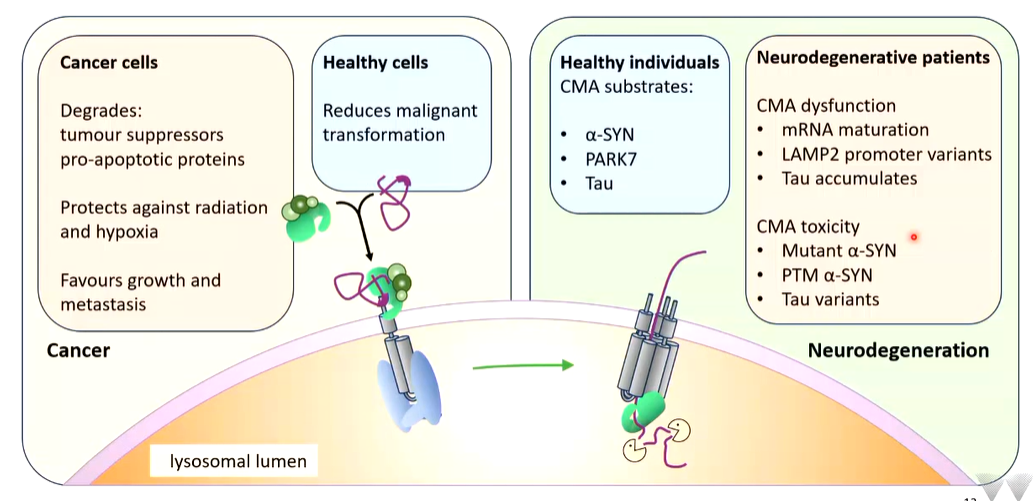
In healthy individuals, substrates do not form as they are removed by CMA.
Microautophagy
This is the direct delivery - lysosome engulfs the target by invagination or protrusion Late endosomes can also do this and invaginate proteins. Some substrates for this have KFERQ motifs and are delivered by HSC70 by direct binding of the chaperone to phosphatidylserine in the late endosome membrane. The autophagic cargos are degraded in the endosomal or lysosomal lumen.
This underlines the central roles of HSC70 in proteins quality control
Macroautophagy
This is an active area of research. What id presented is lowkey an overview
Digestive lysosomes
Lysosomes when are fused with phagosomes or autolysosomes are made into digestive lysosomes that tabulates following incision and regenerates lysosomes. The cargo getting in doesn’t matter,
There are four things to consider in terms of autobiography:
controlled by the ER
Initiation
There is delivery for CMA via LAMP2A (process described earlier) and supplement with growth factors turns CMA off. mTORC2 via activation of AKT1 activates mTORC1. They look similar as they are complexes of proteins but mTORC1 has raptor while mTORC2 has rictor.
mTOC stands for mammalian/mechanistic target of rapamycin and are kinases. mTORC1 phosphorylates members of the ULK complex which inactivates them. This inhibits macroautophagy as well as CMA
Starvation stimulates autophagy and macroautophagy. Withdrawing nutrients and growth factors means that phosphorylation of ULK is decreased. ATG13 was phosphorylated by mTORC14 that inhibited ULK (regulatory in this complex).
Interference with rapamycin can interact with mTORC1 which destabilized it and stops the kinase activity - pharmacological intervention. This also stimulates autoimmune diseases
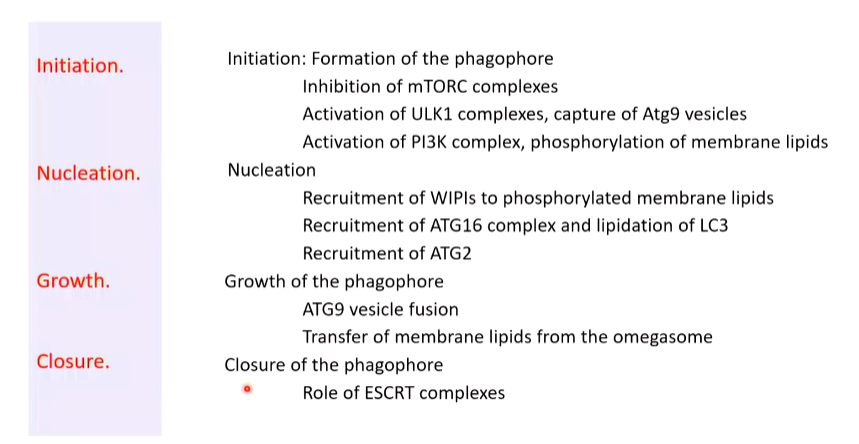
ATG13 captures vesicles that contain ATG9 in their membrane. The ULK1 complex associate on the ER membrane and capture these vesicles. On these vesicles there are associated PI3K complexes with a regulator called BECLIN. ULK1 phosphorylates this that activates PI3K that phosphorylates some membrane lipids of the captured vesicles.
ATG9 is a protein required for maintenance of membranes. It makes sure there is an equal amount of lipids on both sides. Captured vesicles come from multiple sources
nucleation
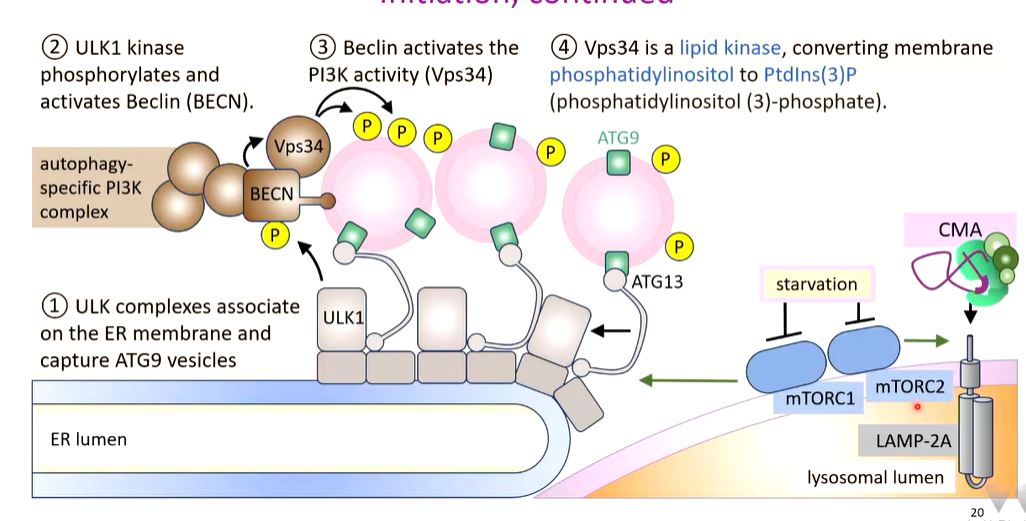
The association of vesicles and complexes forms a complex called the omegasome which may be an ERES. Phosphorylated lipids on ATG9 vesicles capture WIPI proteins which capture further complexes such as ATG16L1 that takes a protein called LC3-1 and attaches it to some membrane lipids in specific covalent attachments (LC3-II).
The other WIPI captures ATG2 that makes intimate contacts with the captured vesicles and the omegasome.
Vesicle fusion
The vesicles now fuse together and the start of the isolation membrane is formed. Once this is started, it expands rapidly to form a phagophore. Expansion occurs where the contact of ATG2 with the omegasome is
The lipid synthesis site in a site is the ER that are tunneled into the omegasome and through ATG2 wich has a hydrophobic groove. This delivers them to the outer leaflet of the phagophore. ATG9 is a flippase that flips lipids so that both inner and outer leaflets of the membrane expands with ER derived lipids. This leads to expansion to a phagophore.
Closure
The ends of the phagosomes are associated with ESCRT proteins that bridges the gap. ESCRT III forms a plug that is coordinated by ULK. Complexes of proteins organise this process. The membranes are bought together and fuse
summary
The importance of LC3
These are also used for selective processes
There are 3 isoforms of this proteins. The proforms are cleaved proteolytically after lysine to form the mature product. On demand, a proportion of LC3-1 are directed to the growing phagophore and attached to PE to generate LC3-2
This process is amplified in selective processes
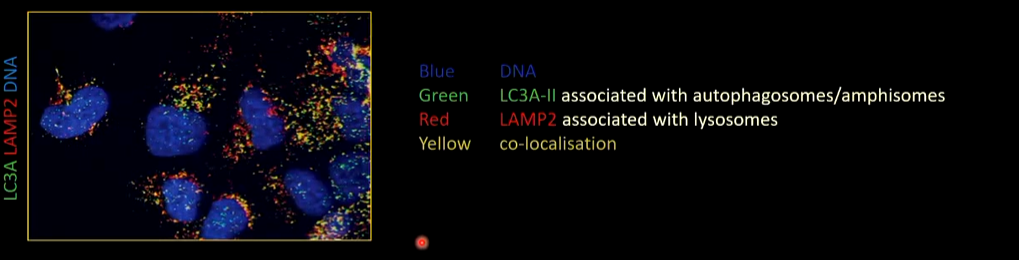
There is a lot of co-localisation where both LC3-II and LAMP2 associate. This means that autophagosomes and amphisomes fuse with lysosomes.
There is no co-localisation with LC3A-II and LC3B-II associate with different vesicles as there is no co-localisation
LC3C-II localised to different membranes
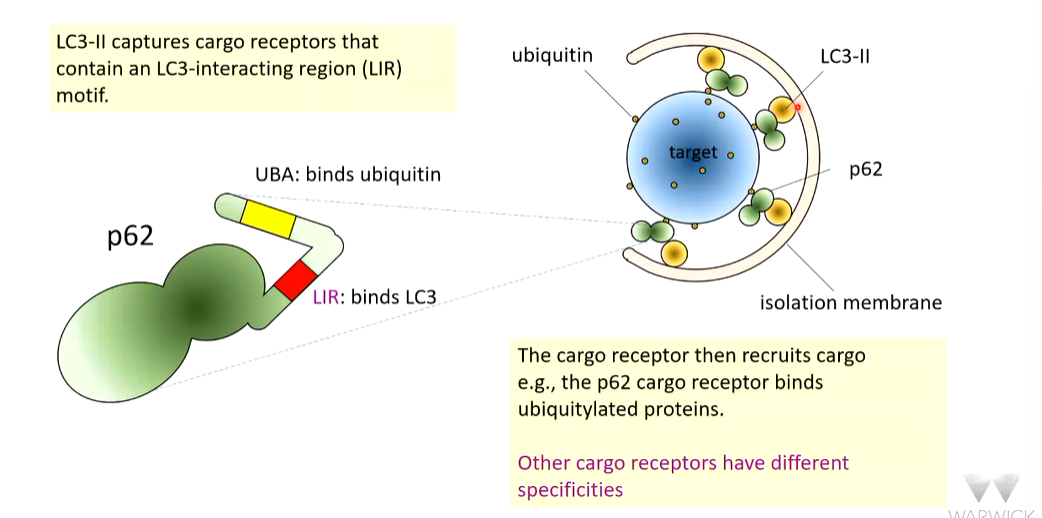
LC3-1 in the cytosol can capture cargo. If the cargo is destined for destruction, P62 can identify this and associate with LC3 and stimulated it capture by ATG16.
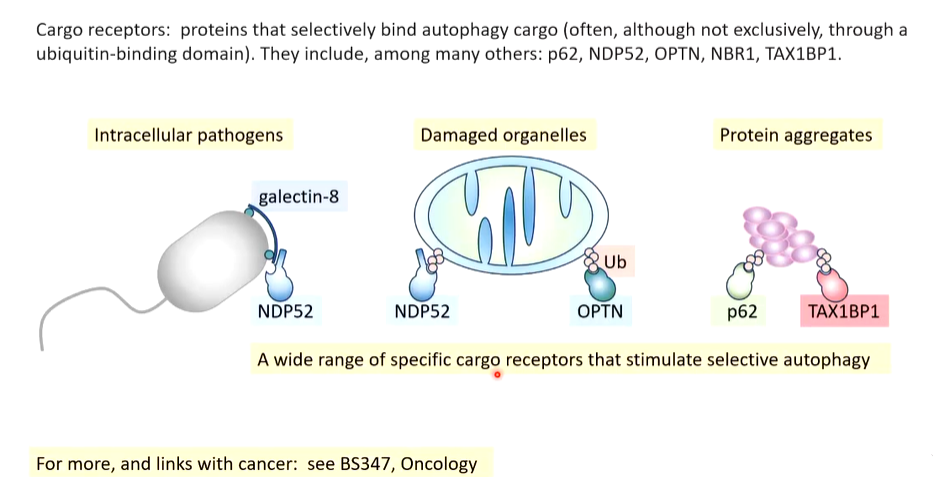
Cargo receptors
How does LC3-II capture receptors
for the exam: