ap bio unit 3 review
Enzymes
Enzyme Structure and Function
Structure Overview
enzymes: biological catalysts; used to speed up biological processes
- ribozymes: biological catalysts made of RNA
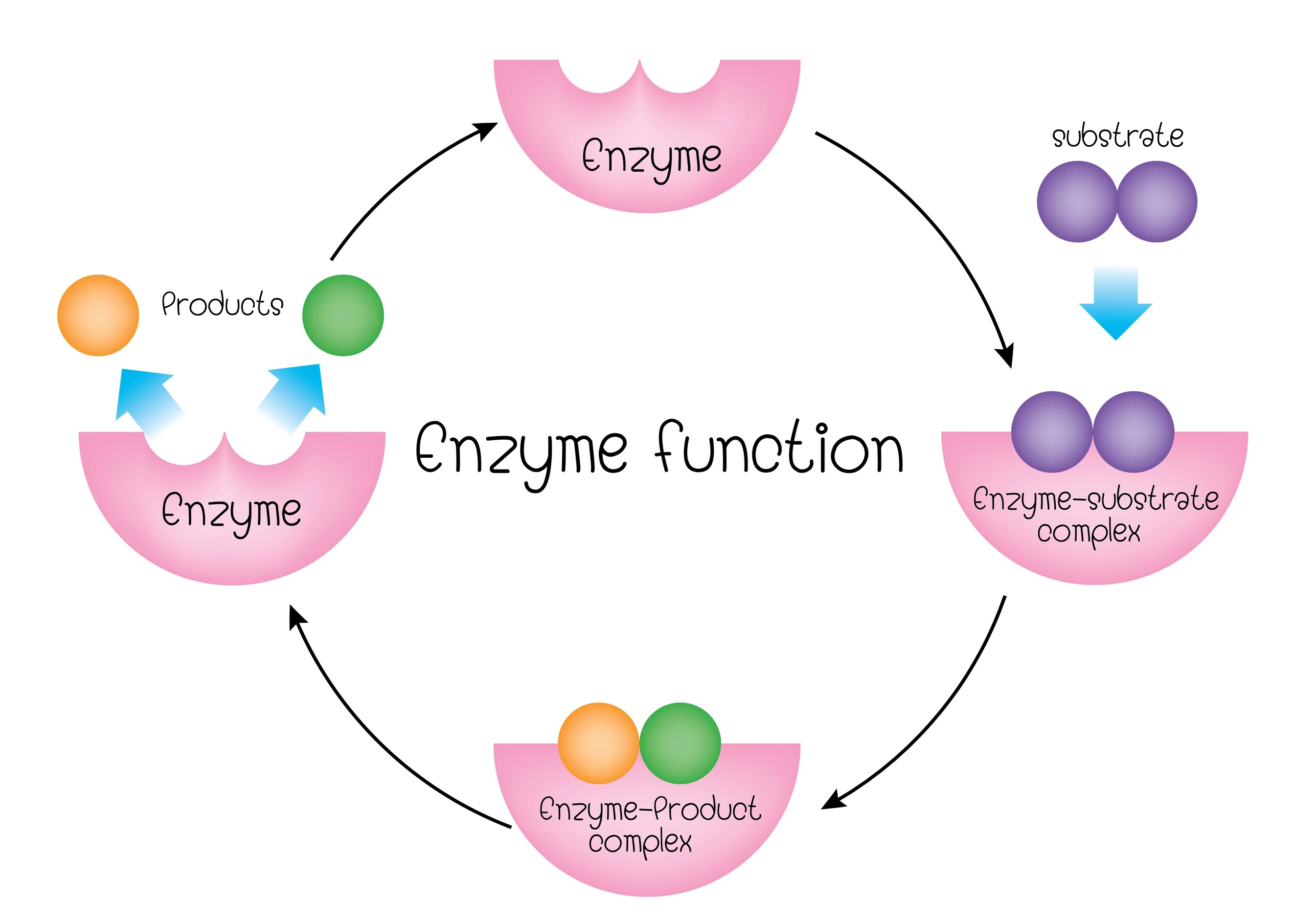
active site: this part of an enzyme interacts with the substrate (reactant)
- shape of the active site on an enzyme is specific to the enzyme and its function
- substrate and active site shapes must match in order for enzyme to work
- any charged r-groups on the amino acids within an active site of an enzyme must have compatible charges on the substrate
Environmental Factors that Affect Enzyme Functions
enzymes catalyze at the most optimal temperature and pH levels
- temp. is too low = less frequent collisions between enzyme and substrate; reaction slows down
- temp. is too high = enzyme can denature; bonds between substrate and enzyme can be affected; enzyme shape can alter
- pH levels are different = bonds in enzyme can be disrupted and tertiary structure of enzyme can change
denaturation: changes to an enzyme’s structure; can limit an enzyme’s ability to catalyze chemical reactions
- denaturation can sometimes be reversed if the reaction returns to optimal conditions
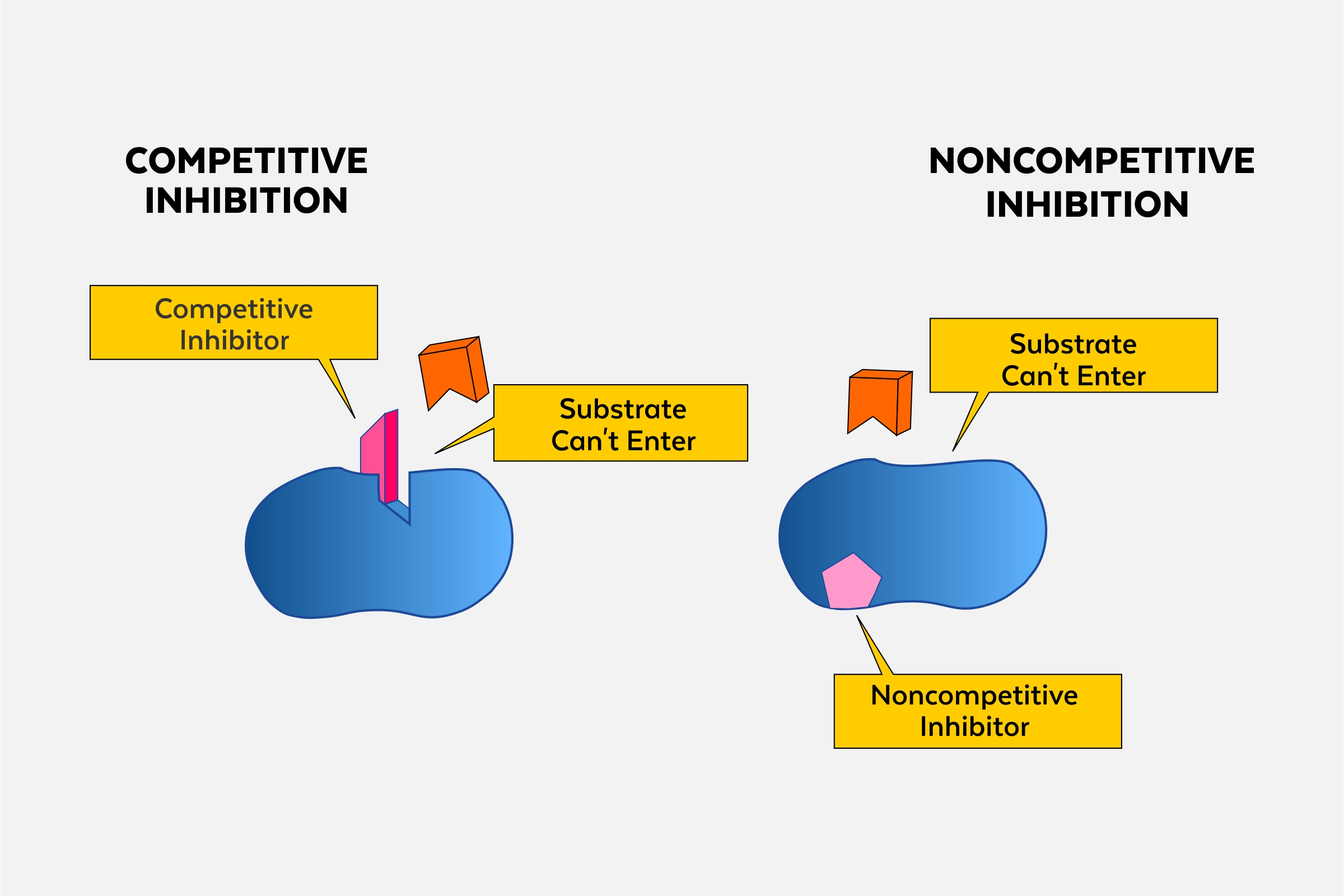
competitive inhibitors: compete with substrates for the active site of the enzyme
- competition lowers the rate of enzyme-substrate reactions occurring
- effects of competitive inhibitors can be reduced by increasing the concentration of the substrate (reactant)
non-competitive (allosteric) inhibitors: do not bind to active site and they bind to a different site on the enzyme; the binding changes the shape of the enzyme, which changes the shape of the active site and reduces the amount of enzyme-substrate bonding
- higher concentration of substrate does not affect non-competitive inhibitors
- non-competitive inhibitors function in feedback mechanisms by adjusting the rate of chemical reactions in the cell to suit changing environmental conditions
cofactors: inorganic molecules and coenzymes (organic molecules): increase the efficiency of enzyme-catalyzed reactions
- they usually increase efficiency by binding to the active site or the substrate to enhance the binding of the substrate to the active site
Activation Energy in Chemical Reactions
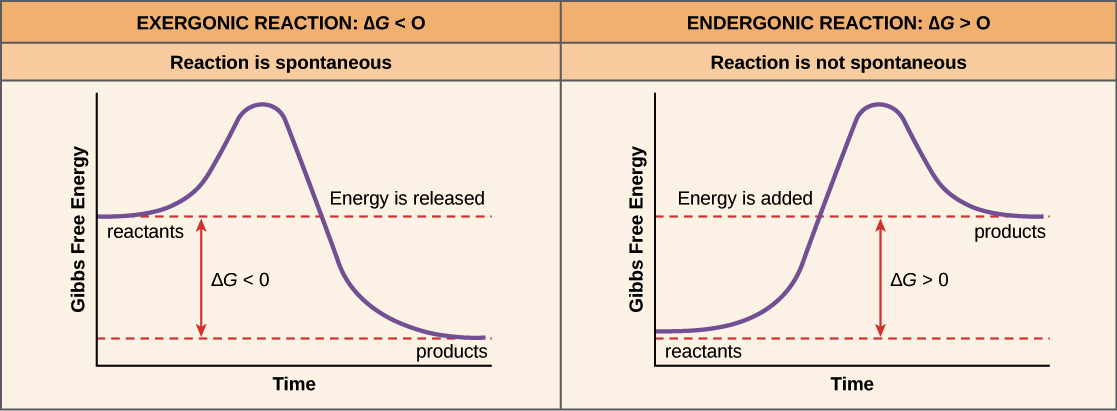
- all molecules have a given amount of free energy (G); chemical reactions in biological processes involve changes in molecules
- chemical reactions can be endergonic or exergonic
- endergonic reaction: has products with higher free energy levels than its reactants; considered energetically unfavorable
- exergonic reaction: has products with lower free energy levels than its reactants; considered energetically favorable
- all chemical reactions require an input of energy to reach a transition state/start the reaction
- activation energy (Ea): the difference between the energy level of the reactants and the energy level of the transition state of the reaction
- higher Ea: results in slower rate of chemical reactions
- lower Ea: results in faster rate of chemical reactions
- enzymes can lower activation energy (Ea) in multiple ways:
- bringing the substrates together in proper orientation for a reaction to occur
- destabilizing chemical bonds in the substrate by bending the substrate
- forming temporary ionic or covalent bonds with substrate
- enzymes can lower activation energy but they cannot change an endergonic reaction (energetically unfavorable) to an exergonic reaction (energetically favorable)
Energy and Metabolism/Coupled Reactions
- energy input into cell must be higher than energy requirements for cellular systems
- processes that release energy can be paired (coupled) with processes that require energy
- coupled reactions: occur in multiple steps to allow for the controlled transfer of energy between molecules (leads to more efficiency)
- coupling exergonic reaction with endergonic reaction: allows the energy released by the exergonic reaction to “drive” the endergonic reaction
- example:
- exergonic step: breakdown of ATP into ADP and a phosphate group (Pi (inorganic)); releases approx. 30 kilojoules of energy per mole of ATP
- endergonic reaction: the reaction that combines glucose with fructose to form sucrose requires approx. 27 kilojoules of ATP per mole of sucrose formed
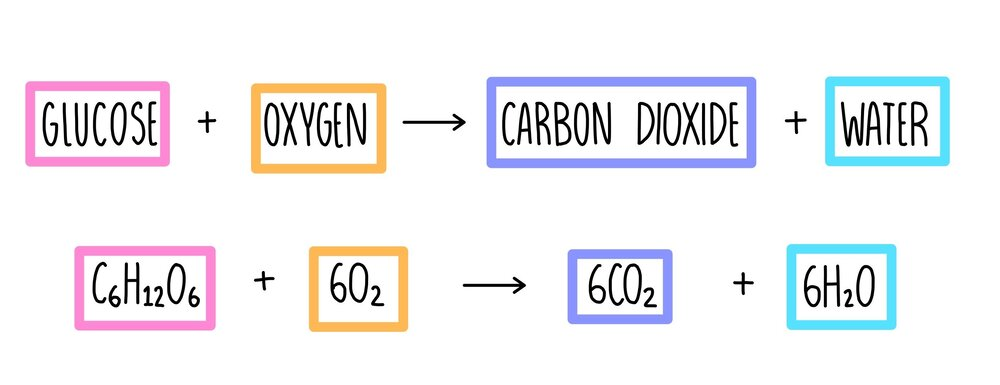
Photosynthesis
Overview
heterotrophs: consume other organisms to obtain energy required for biological processes
autotroph: produce their own organic molecules from inorganic molecules
- photo-autotrophs: autotrophs that use light energy to power the process
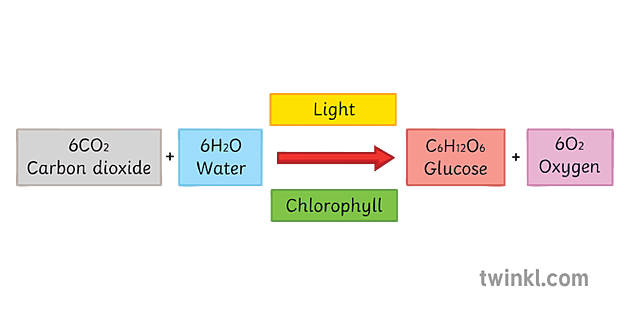
- photo-autotrophs: autotrophs that use light energy to power the process
light-dependent reactions: use energy from sunlight to split water to produce oxygen gas, protons, and high-energy electrons
- oxygen gas: released into atmosphere
- protons and high-energy electrons: used to power the production of ATP and NADPH
- ATP and NADPH are sent to the light independent reactions
light-independent reactions: use ATP, NADPH, and carbon dioxide to produce sugars
- ATP, Pi (inorganic phosphate group), and NADP+: sent back to light-dependent reactions
plants: photosynthesis occurs in the chloroplasts
- chloroplasts:
- outer membrane: filled with liquid called stroma
- stroma: has floating stacks of membranous sacs called grana; location of light-independent reactions
- thylakoid: each individual membranous sac from the grana stack; location of light-dependent reactions
prokaryotes: also perform photosynthesis (ex. cyanobacteria); do not contain chloroplasts
- light-dependent reactions: occur on infoldings of plasma membrane
- light-independent reactions: occur in the cytosol
Light-Dependent Reactions
- photophosphorylation: conversion of ADP to ATP using the energy of sunlight by activation of PSII
- light energy excites the electrons in the chloroplasts to a higher energy level; energy is released as excited electrons move through chloroplasts
- NADP+ accepts the electrons to form NADPH
- NADPH: source of reducing power for light-independent reactions
- chlorophyll: light-absorbing pigment that captures the energy of photons from the sun; found in photosystems 1 and 2
- photosystem: composed of proteins, chlorophyll, and accessory pigments; PSI and PSII contain different types of chlorophyll that absorb most light energy at slightly different wavelengths (PSI = 700 nm; PSII = 680 nm)
- photosystems are located in the thylakoid membrane of the chloroplast; they are connected by an electron transport chain (ETC)
- accessory pigment: other light-absorbing pigments besides chlorophyll
The Process of Light-Dependent Reaction
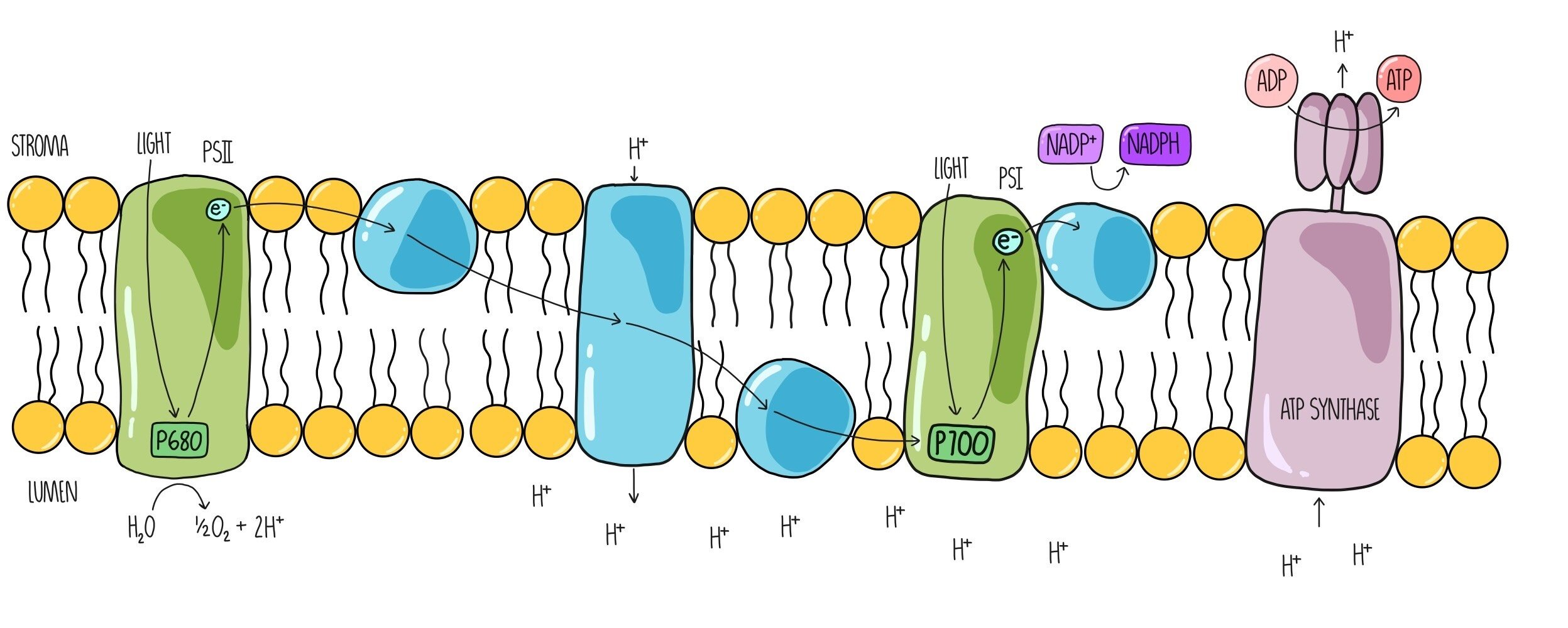
- energy in p+ is used to boost e- in chlorophyll to higher energy level in PSII
- e-s from PSII are passed from one protein carrier to another in a series of redox reactions (like falling down a hill)
- the final e- donor in the ETC passes the e- to the PSI
- as the e-s pass through the ETC, energy is released and used to create a proton (H+) gradient
- H+ ions are actively transported against concentration gradient across the thylakoid membrane
- electrons from PSII that fell down the ETC are now in PSI and need to be replaced in PSII
- photolysis: the splitting or decomposition of a chemical compound by means of light energy or photons
- occurs when splitting of water molecules takes electrons from hydrogen atoms and produces H+ ions, electrons from PSII, and oxygen gas
- proton gradient generated by photolysis of water and the ETC powers the production of ATP by ATP synthase
- chemiosmosis: process of using a proton gradient and ATP synthase to produce ATP; also used in mitochondria to generate ATP during cellular respiration
- the electron from the ETC that is now on PSI is boosted by a photon of light energy from the sun; the electron passes again through a series of carriers (much shorter than ETC) where it is finally transferred (along with a proton) to NADP+ by the enzyme NADP+ reductase
- this produces a molecule of NADPH, which will provide the reducing power for the light-independent reactions
Light-Independent Reactions (Calvin Cycle)
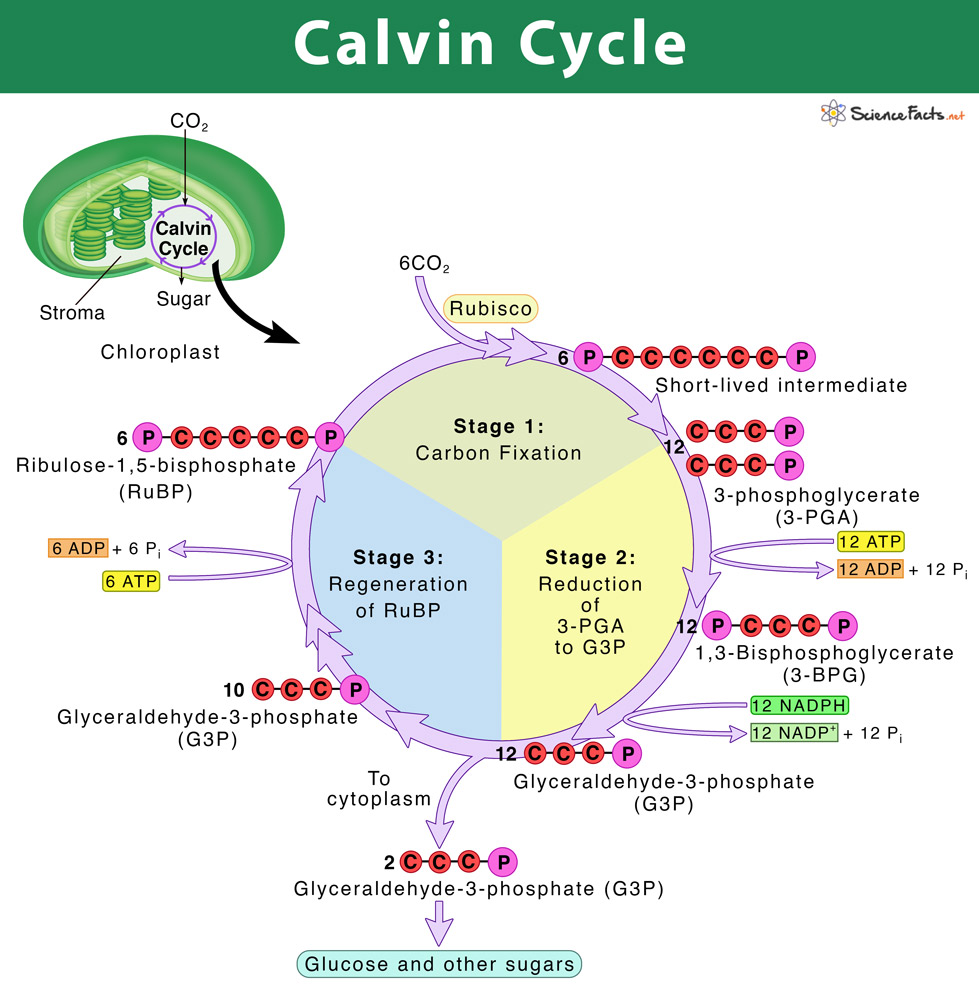
- light-independent reactions occur in stroma of chloroplast
- the process can be broken down into 3 part:
- fixation of carbon
- reduction
- regeneration of RuBP
Fixation of Carbon
- fixation: to turn a biologically unusable form to a usable form
- enzyme ribulose-biphosphate-carboxylase (rubisco) adds one molecule fo carbon dioxide to the 5-carbon molecule ribulose-biphosphate (RuBP)
- this produces a 6-carbon intermediate that is unstable
- the unstable molecule is then broken down further into two 3-carbon molecules
Reduction
- the ATP and NADPH from the light-dependent reactions are used to reduce the 3-carbon molecules into glyceraldehyde-3-phosphate (G3P)
- G3P: can be used to make sugars; some of it is used during regeneration
Regeneration
- 5-carbon RuBP must be regenerated for the calvin cycle to continue
- for every 5 molecules of G3P, there are 15 carbon atoms present
- ATP from light-dependent reaction in used to rearrange the five G3P (3-carbon molecule) molecules to form 3 molecules of RuBP (5-carbon molecule)
- this process requires energy that comes from the light-dependent reactions
Cellular Respiration
Overview
- cellular respiration includes the following processes:
- glycolysis
- oxidation of pyruvate
- krebs cycle (citric acid cycle)
- oxidative phosphorylation
- the presence of oxygen determines whether the process will be anaerobic or aerobic
- anaerobic: without oxygen; anaerobic organisms (don’t have access to oxygen) can perform glycolysis and fermentation
- aerobic: with oxygen; aerobic organisms (have access to oxygen) can perform all processes in the presence of oxygen and only glycolysis and fermentation in the absence of oxygen
- can perform more processes than anaerobic, meaning they can extract more energy from organic compounds
| cellular process | anaerobic organisms (without oxygen) | aerobic organisms (with oxygen) | aerobic organisms (without oxygen) |
|---|---|---|---|
| glycolysis | ✓ | ✓ | ✓ |
| oxidation of pyruvate | ✓ | ||
| krebs cycle | ✓ | ||
| oxidative phosphorylation | ✓ | ||
| fermentation | ✓ | ✓ | ✓ |
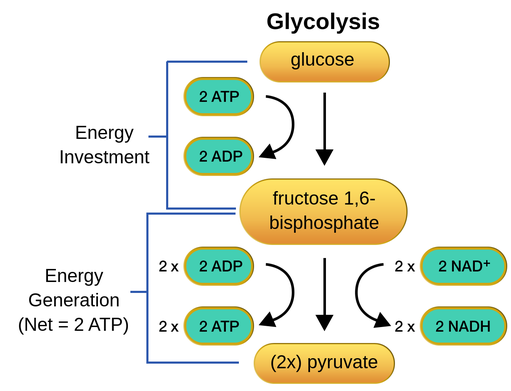
Glycolysis
- occurs in the cytosol of the cell (all organisms have cytosol, so all organisms can perform glycolysis)
- a 6-carbon molecule enters glycolysis alone with two molecules of NAD+ (electron carrier)
- each NAD+ is reduced (loses hydrogen atom and electrons) to NADH
- two molecules of ATP are required for early steps of glycolysis
- four molecules of ATP are produced by glycolysis (net gain of ATP)
- at the end of glycolysis, the 6-carbon glucose molecule is split into two 3-carbon pyruvate molecules
| location | inputs | outputs |
|---|---|---|
| cytosol | glucose (6C) | 2 pyruvate (3C) |
| 2 NAD+ | 2 NADH | |
| 2 ATP | 4 ATP |
Oxidation of Pyruvate
occurs in the mitochondria
the 3-carbon pyruvate molecule is oxidized (loses hydrogen atom and electron) and NAD+ is reduced (gains a hydrogen and its electrons) to become NADH
- as this happens one of the carbons of the pyruvate molecule is reduced as CO2 (leaving behind a 2-carbon acetyl group)
coenzyme A attaches to the 2-carbon acetyl group and delivers the acetyl group to the krebs cycle
each molecule of glucose that enters glycolysis generates 2 pyruvate so oxidation of pyruvate occurs twice for each molecule of glucose
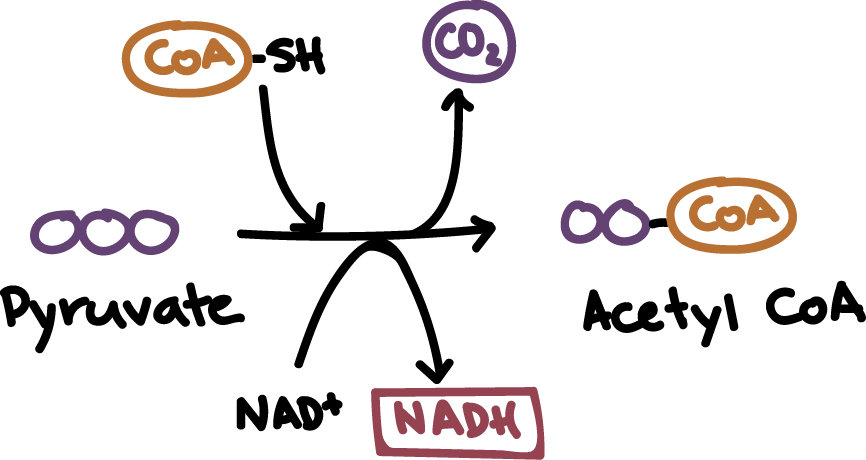
| location | inputs | outputs |
|---|---|---|
| mitochondria | pyruvate (3C) | acetyl group (2C) |
| NAD+ | carbon dioxide (1C) | |
| NADH |
Krebs Cycle (Citric Acid Cycle)
occurs in the matrix (liquid center) of the mitochondria
coenzyme A brings the 2-carbon acetyl group to the cycle (initially attached as 4-carbon intermediate but forms 6-carbon molecule)
the 6-carbon molecule goes through a series of enzyme-catalyzed reactions to regenerate the 4-carbon intermediate (and produces 2 molecules of CO2)
at the end, all the carbon that was originally in the glucose molecule (at the start of glycolysis) has been released as CO2
during the cycle:
- one molecule of ATP is produced from substrate-level phosphorylation
- phosphorylation: direct addition of a phosphate group to ADP without the use of an electron transport chain or chemiosmosis
- three molecules of NAD+ are reduced to NADH
- one molecule of FAD+ is reduced to FADH2
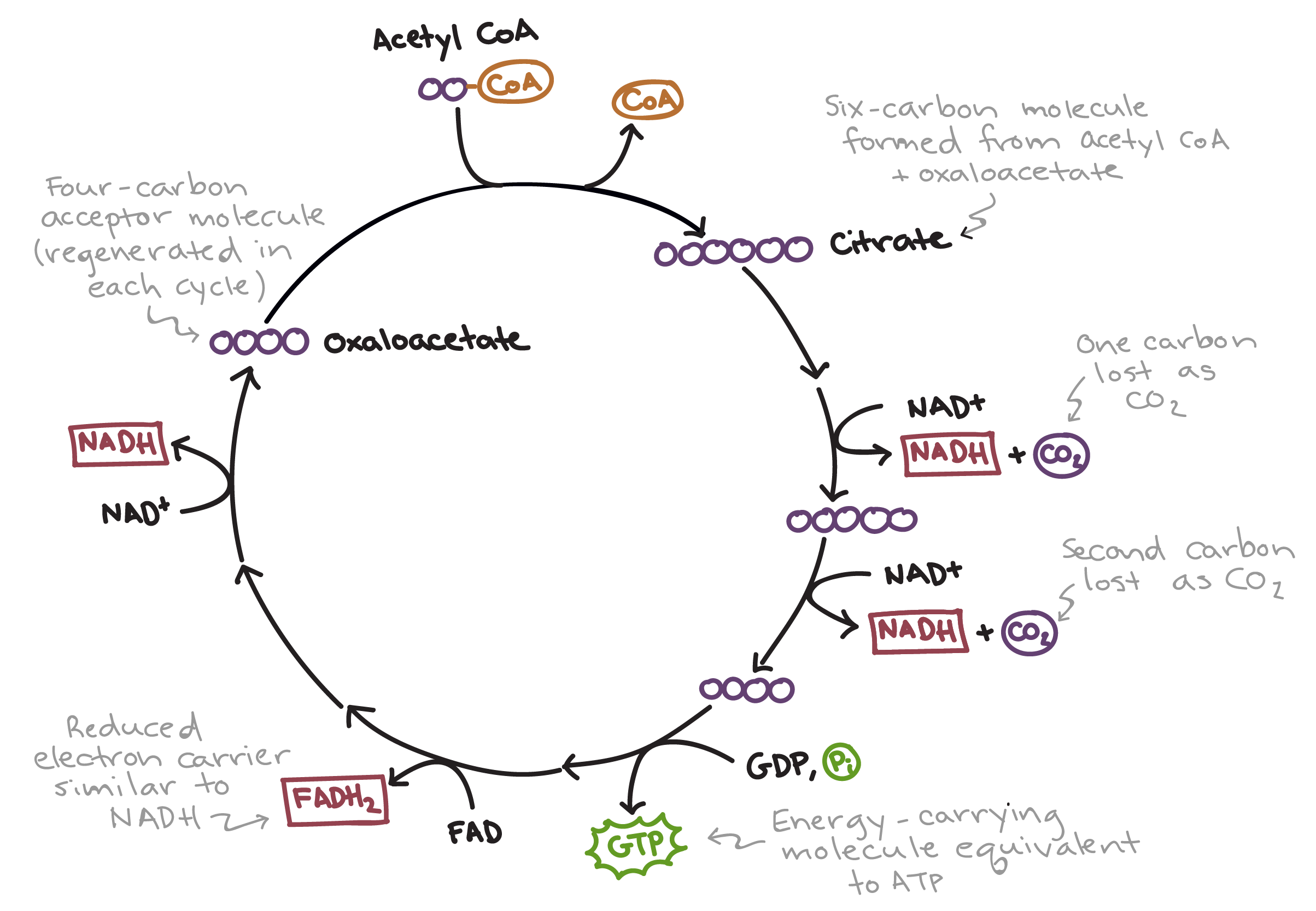
| location | inputs | outputs |
|---|---|---|
| matrix of mitochondria | acetyl group (3C) | 2 carbon dioxides (1 C each) |
| 3 NAD+ | 3 NADH | |
| 1 FAD+ | 1 FADH2 | |
| 1 ADP + Pi | 1 ATP |
Total Products of Glycolysis, Oxidation of Pyruvate and Krebs Cycle for Each Molecule of Glucose
| molecule | glycolysis | oxidation of pyruvate | krebs cycle | total |
|---|---|---|---|---|
| ATP | 2 (net) | 0 | 2 | 4 |
| NADH | 2 | 2 | 6 | 10 |
| FADH+ | 0 | 0 | 2 | 2 |
| CO2 | 0 | 2 | 4 | 6 |
- all 6 carbons in glucose molecule have been released as CO2
- four molecules of ATP have been produced by substrate-level phosphorylation
- a total of 12 high-energy electron carriers (10 NADH and 2 FADH2) have been produced and will enter oxidative phosphorylation
Oxidative Phosphorylation
involves the ETC and chemiosmosis (both of which occur in the membrane of the mitochondria)
this process yields the most production of ATP in cellular respiration
the electron carriers (NADH and FADH2) previously produced carry their electrons to the ETC
- as they deliver their electrons they are oxidized to NAD+ and FAD+, which can be used earlier on in cellular respiration
- as the electrons travel through the ETC their PE decreases and energy is released
the released energy is used to pump H+ out of the matrix and int the intermembrane space of the mitochondria to create a proton gradient
at the end of the ETC, molecular oxygen (O2) combines with four protons (H+) and four electrons (e-) to form 2 water molecules (2H2O)
- this means oxygen is the final (terminal) acceptor of electrons in cellular respiration
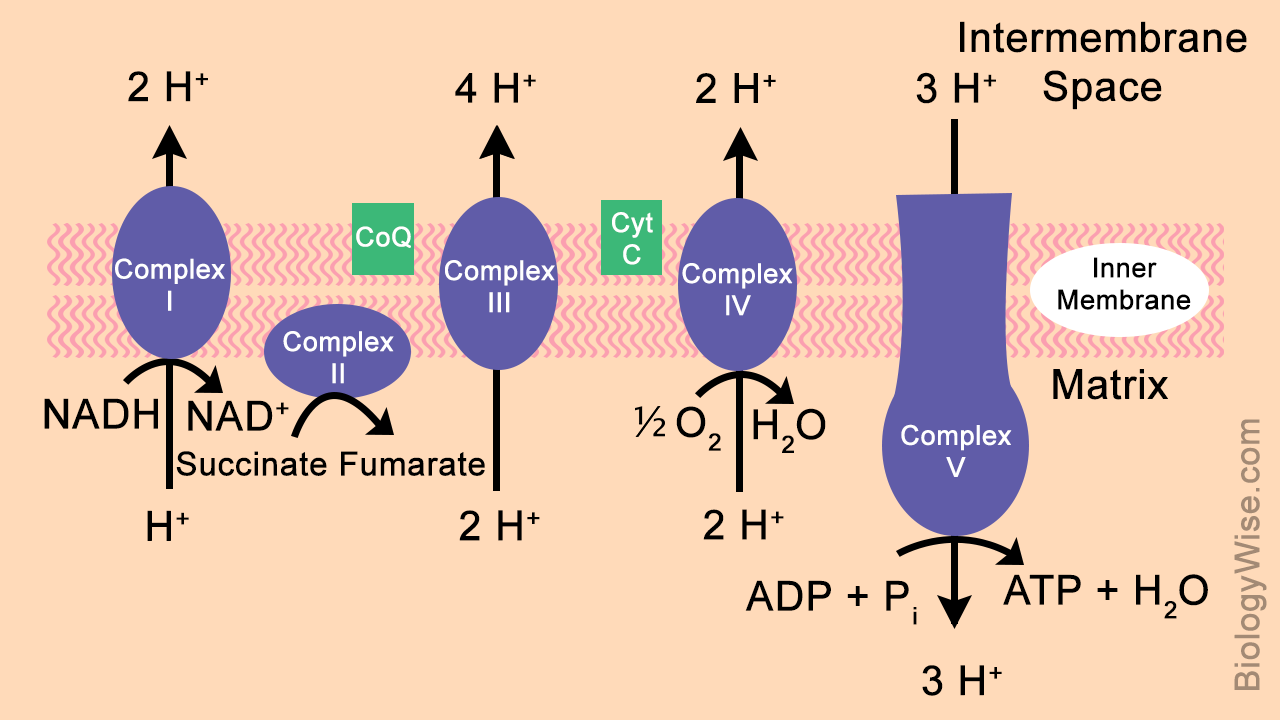
the proton gradient created by the ETC is used to produce ATP through chemiosmosis
- protons flow from areas of higher concentration in intermembrane space to areas of lower concentration in the matrix through a channel in the ATP synthase enzyme
- this flow of protons leads to a change in the shape of the enzyme; the new shape of the enzyme allows for ATP synthase to catalyze the production of ATP
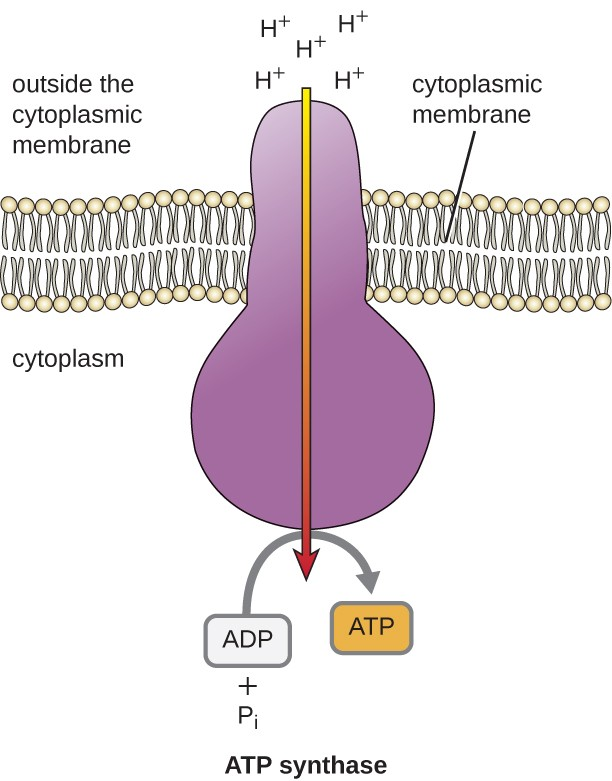
(ideally) 34 total ATP molecules can be produced:
- 10 NADH x 3 ATP = 30 ATP
- 2 FADH2 x 2 ATP = 4 ATP
- FADH2 has a lower potential energy than NADH, so it produces less ATP
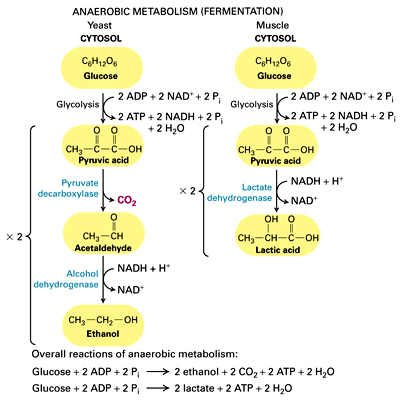
Fermentation
when oxygen isn’t present, oxidative phosphorylation cannot occur (oxygen is the final electron acceptor)
anaerobic conditions require the use of fermentation to regenerate NAD+ needed to keep the process of glycolysis going
fermentation only occurs in the cytosol
alcohol fermentation: pyruvate is reduced to an alcohol (usually ethanol) and CO2; NADH is oxidized to NAD+
- example: yeast undergoing fermentation for bread to rise
lactic acid fermentation: pyruvate is reduced to lactic acid (3-carbon molecule); NADH is oxidized to NAD+; no CO2 is produced
- example: can occur in muscle cells if they do not have enough oxygen to carry out oxidative phosphorylation