Physio III (Endocrinology and Reproduction) Lecture
Introduction to Endocrinology
Endocrinology is the study of communication within organisms by means of hormones
Hormones are the chemical messengers of the endocrine system
ex. testosterone, melatonin, serotonin, insulin, glucagon, gastrin, vasoactive peptides
The discipline of endocrinology includes:
study of hormones
anatomy and physiology of cells, tissues & organs that produce these hormones.
the way hormones are transported & act on target cells
there’s a specificity of each hormone
clinical abnormalities of hormonal deficiencies & overproduction
hypothyroidism - skin problems (T3,T4 &TCH)
clinical signs: skin lesions similar with external parasites
overproduction of growth hormone > gigantism
deficiency of GH > dwarfism
Hormone - chemical substances released by cell/gland in 1 part of the body that sends out messages that affect cells in other parts of the organism.
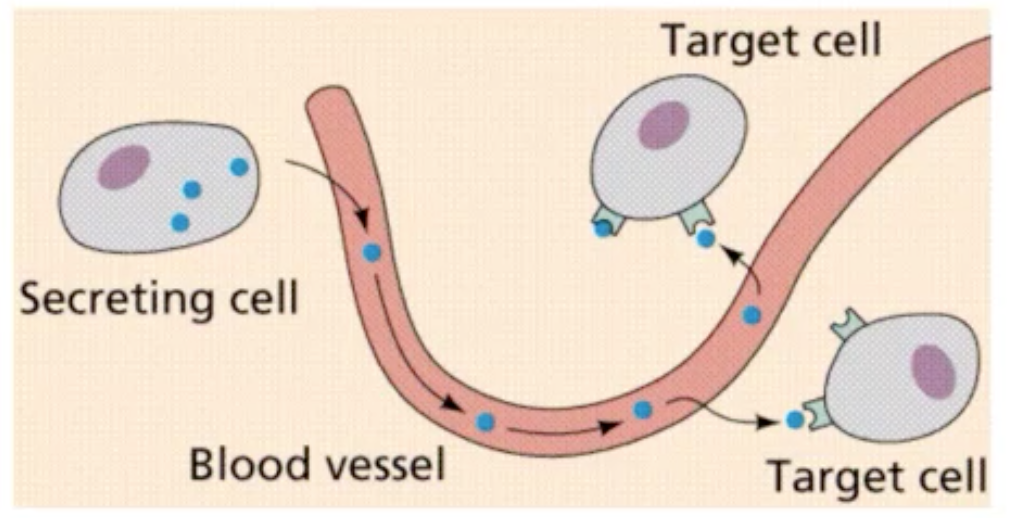
In classical sense, hormone was described as chemical messenger secreted from ductless gland, emptied directly into the circulation and transported by the blood. (hemocrine/endocrine communication - some distance to alter the function of target organ)
In today’s definition, this blood-borne communication is one manifestation of the endocrine system.
The concept ductless glands are sole sources of hormones has gone by the wayside bc hormones are also secreted throughout the body by nonglandular tissues whose major function are primarily nonendocrine.
They can also be transported not only by the blood; There are other routes wherein these hormones can be transported other than the circulation.
Components of Endocrine system (other than blood vessels)
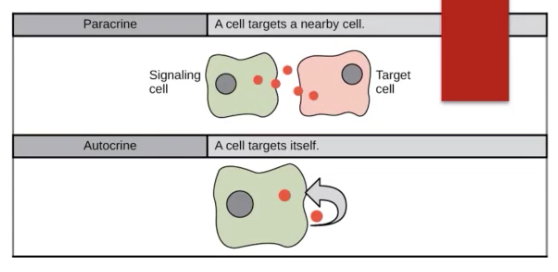
autocrine communication - hormones are secreted locally to the extracellular fluid only to return to self regulated cells that arranged them.
The released hormone will return to the same cell that released the hormone to self regulate.
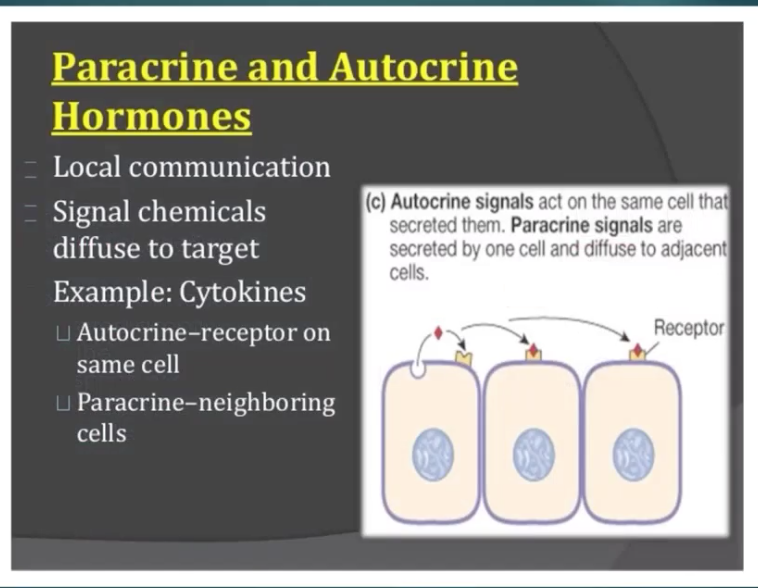
paracrine communication - secretion of hormones from cell directly into the surrounding ECF. Hormones interact with adjacent/nearby cells without being transported by blood. It delivers very high concentration of hormone to its target site.
signaling of cells and it releases into the ECF > into the target cells
The concentration of hormones is HIGH
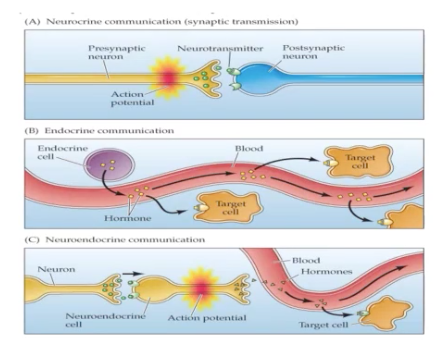
neurocrine communication - secretion of peptides or other neurotransmitter moleceules by neurons & specialized form of paracrine function in which the chemical messenger is transferred to a target cell via synapse or neuromuscular junction.
There are hormones that belong to the peptide hormones and they are considered as hormones.
Neurocrine = Synapse or synaptic transmission
Presynaptic neuron will release neurotransmitter due to the action potential going to the Postsynaptic neuron
Acetylcholine/Ach - Most popular neurotransmitter
Neuroendocrine communication - AKA hemocrine communication — it is released in the blood & moves into the target cell
No postsynaptic neuron
W/ synapse > will go directly to the blood & into the target cell
solinocrine - hormones like gastrin, somatostatin, vasoactive peptide, calcitonin, secretin & serotonin are secreted directly into the lumen of gastrointestinal, respiratory & reproductive tract.
Where hormones secreted they will go directly into the target organ.
Other types of signaling:
intracrine
intercrine
juxtacrine
solinocrine
Function of hormones
single hormone can affect a single function
ex. erythropoietin on hemoglobin synthesis by erythrocytes — but this one function is very rare
erythropoietin is hormone responsible for erythropoiesis
kidney - organ produces erythropoietin
actions involving single hormones having multiple actions
ex. thyroxine on enzyme synthesis, erythropoiesis, bone turnover, carbohydrate & lipid metabolism
thyroxine / T4 / tetraiodothyronine
multiple hormones having single actions
ex. regulation of lactation/lactogenesis by prolactin, placental lactogen, corticosteroids, thyroxine, sex steroids & oxytocin.
multiple hormones having multiple actions
reproductive steroids (estrogen, progesterone), oxytocin & corticosteroids on pregnancy, fetal development and parturition
progesterone - responsible for the maintenance of pregnancy
estrogen - responsible for the preparation
near birth > progesterone will be blocked > initiation of parturition > prostaglandin
from puberty age > cyclical activity of reproductive hormones (FSH, LH) > target organ is reproductive system (ovary) > development of sexual characteristics and behaviors
reproduction depends on the effects of hormones on gametogenesis
spermatogenesis - formation of sperm, testosterone
oogenesis - primary egg to ovum
development of sexual characteristics and elicitation of behaviors that culminate in the fertilization of an oocyte by a spermatozoon and production of offspring
Many hormones play important function for maintenance of pregnancy including growth of embryo and fetus, development of reproductive tract for pregnancy and initiation of parturition.
Once birth has occurred, newborn must independently adapt quickly to the extrauterine environment, respond to stressors and maintain homeostasis in every physiological function.
Hormones & Nervous system are vital for maintenance of the animal’s internal environment.
Hormones and their actions are essential for pre and postnatal growth and development.
Hormones are important in timing the cessation of growth
growth hormone - mediated closure of epiphyses of long bones after maturity is necessary for proper adult body conformation.
Stop of growing bc of the closing of the epiphyseal plate of long bones
Production, storage and utilization of energy require complex-endocrine regulated ingestive, digestive, anabolic, catabolic and excretory processes.
Characteristics of Endocrine System
Players of endocrine system are hormones which are classified into:
lipid
protein
miscellaneous
Protein hormones include:
prolactin
growth hormone
Glycoprotein hormone include:
thyrotropin / thyroid stimulating hormone (TSH)
luteinizing hormone (LH)
follicle stimulating hormone (FSH)
Peptide hormones include:
insulin
insulin-like growth factor-1 (IGF-1)
adrenocorticotropin (ACTH)
Hormones that are derivates of amino acid:
triidothyronine
catecholamines
epinephrine and norepinephrine
Lipidic hormones include:
steroids
ex. progesterone, estrogens, androgens, glucocorticoids and mineralocorticoids
eicosanoids
ex. prostaglandins, thromboxanes & leukotrienes
Whatever chemical nature of hormones, they all have several characteristics in common.
They are present in the blood and other extracellular fluids in low concentrations.
Despite their low concentrations, development and refinements of RIA/radioimmunoassay and other modern quantitative techniques allow routine measurements of hormones in small samples of serum, plasma or urine for research, clinical diagnostic procedure and therapeutic monitoring.
Existence of mechanisms that direct hormones to their target cells and tissues
Hormone — Target cell specificity
Target cells - hormones circulate to all tissues but only activate these cells
They have specific receptors to which hormone binds. These receptors may be intracellular/located on the plasma membrane.
ex. of hormone activity:
ACTH receptors are only found on certain cells of the adrenal cortex
Thyroxin receptors are found on nearly all cells of the body
Cell have high affinity receptors to capture/bind hormones from ECF.
These receptors can reside on the external surface of the cell membrane…..
ex. gonadotropin receptors
Or can be located inside the cell — cytoplasm/nucleus
ex. estradiol receptors
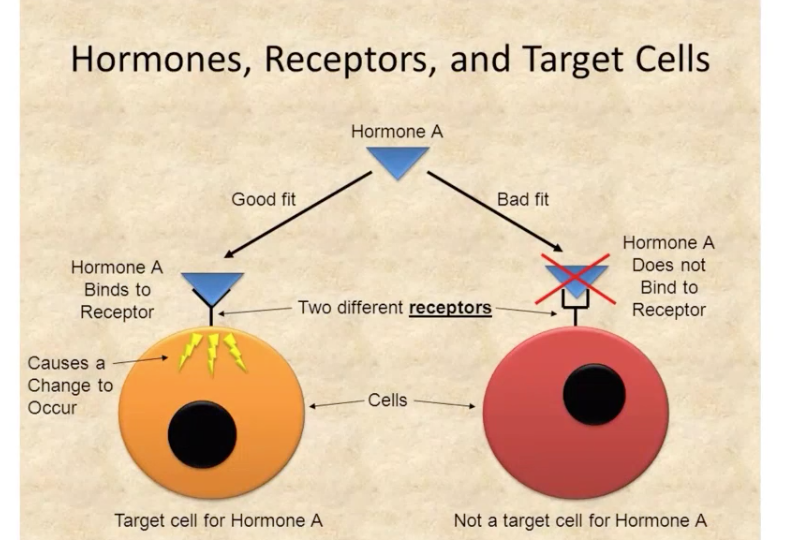
Principal target tissue/s for hormone has greatest concentration of the receptor specific for that hormone.
Some hormones are concentrated in target cells bc of the proximity of the source of hormone to the target cell, allowing direct diffusion of the hormone from its source to an adjacent target cell.
ex. testosterone synthesized by Leydig cells of the testis > diffuses only short distance to Sertoli cells > To adluminal compartment of the seminiferous epithelium to promote spermatogenesis.
Testes
Androgen/testosterone - target organs are body cells which includes testes, skeleton muscles and skin
For the development and maintenance of male secondary sexual characteristics
Its released is controlled by FSH and LH released from anterior pituitary under the control of hypothalamus.
Other hormones are produced within their target cells.
ex. dihydrotestosterone - hormonally active form of testosterone in male and produced by androgen-sensitive target cells such as those of prostate
ex. T4/thyroxine > converted to T3 within the cells of pituitary gland to play major role in regulation of TSH secretion.
1 anatomic feature efficiently directs hormone to their target tissue/s is the portal circulation.
Hormones don’t join the general circulation bc it takes time and much longer route
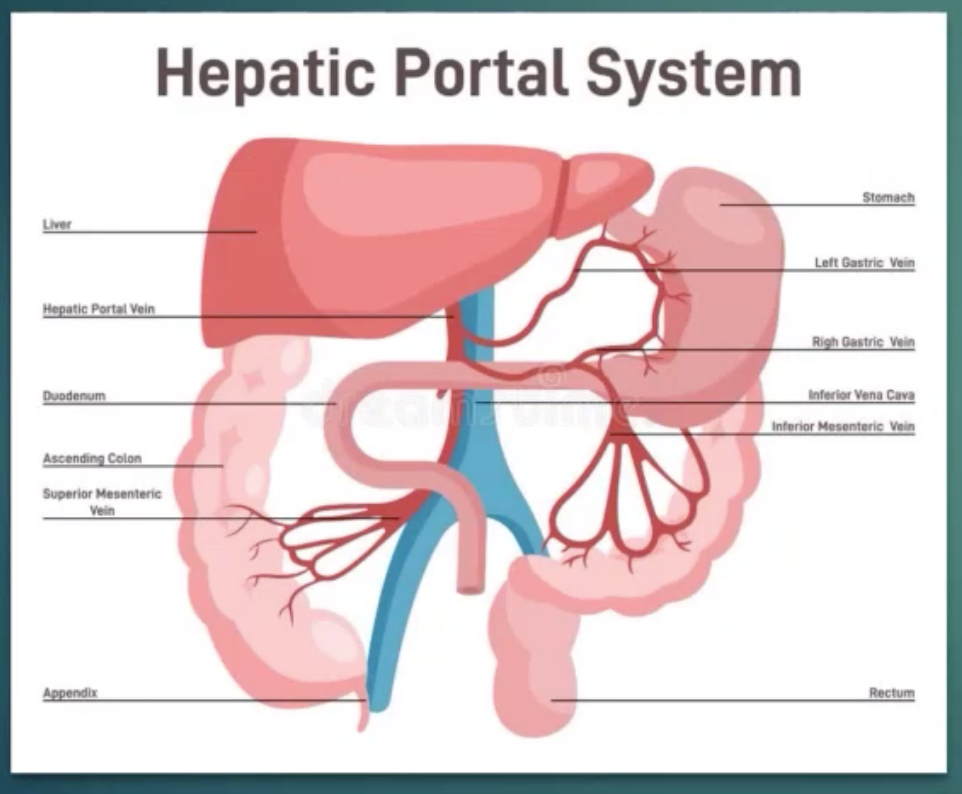
Portal circulation - consists of blood flowing from capillaries in 1 organ to a vein and then to capillaries in another organ.
Hepatic Portal circulation - where insulin is secreted into capillaries of endocrine pancreas and carried by the hepatic portal vein to capillaries of the liver for major actions.
Hypophyseal portal system/circulation - Releasing hormones for hormones of anterior pituitary gland pass from capillaries in the hypothalamus (releasing hormones) to the hypophyseal portal vessels and then to the capillaries of the pituitary gland.
Hormone synthesis & secretion
Peptide & Protein Hormone
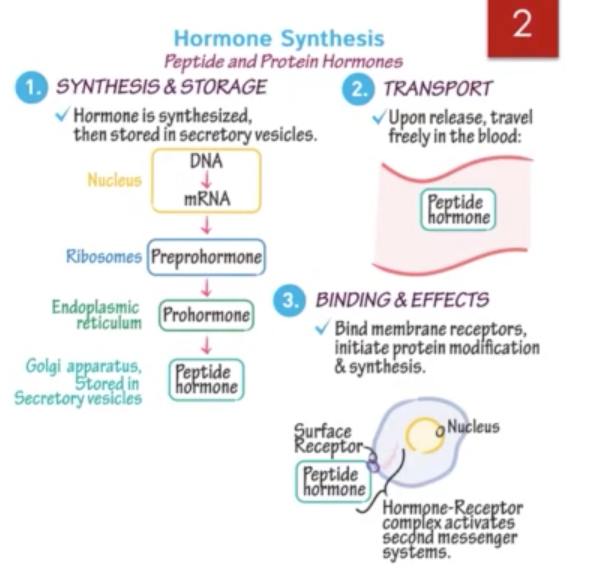
Synthesis of peptide and protein hormones begins with transcription of DNA in the nucleus to yield messenger RNA (mRNA) which encodes prohormone on the rough endoplasmic reticulum.
Animo acids are then polymerized into a peptide into polypeptide prohormone by translation.
Newly synthesized prohormones are released into the cisternae of ER where they are carried to the Golgi complex.
Proteins are processed and packaged into secretory granules/vesicles by budding from ER and golgi membranes.
The immature granules undergo maturation and upon receiving inappropriate extracellular stimulus, the granules and vesicles migrate & fuse with plasma membrane of cell. The processed hormone will then be released into the ECF.
For peptide hormones:
The initial synthetic product is large protein (prohormone) that is cleaved step by step into several peptides, more than one of which have hormonal activity.
Adrenocorticotropin (ACTH) - 39-amino acid peptide derived from much larger precursor (235 amino acids) called propiomelanocortin (POMC)
Other peptides w/ hormonal activity derived from POMC include:
melanocyte - stimulating hormone
beta-endorphin
beta-lipotropin
Parathyroid hormone / PTH - synthesized as part of a large precursor by the chief cells of parathyroid gland.
Parathyroid gland = chief cells
Thyroid gland = follicular cells
The precursor (preproPTH), a polypeptide of 113 amino acids is synthesized on ribosomes of chief cells.
PreproPTH is reduced in the ER to proPTH w/ 90 amino acids
N-terminal hexapeptide then is cleaved within Golgi region to form native PTH w/ 84 amino acids and molecular weight of 9500 daltons
Other prohormones include: proinsulin, proglucagon, progastrin & procalcitonin.
Outdated notion that hormones are synthesized only in specific glands has been challenged often.
ex. complete amino acid sequence of larger gastrointestinal form of glucagon called enteroglucagon, glicentin or glucagon-like immunoreactivity.
Thyroxine - prohormone is converted to hormone T3 in the liver, kidney, brain and pituitary gland.
Characteristics of true endocrine gland that separate it from other organs that also produce hormones are that:
Endocrine glands synthesize hormone at faster rates
Efficiently process prohormones
Have mechanisms for releasing hormone in controlled manner
Usually only small amounts of hormones are stored by endocrine glands. Therefore, secretion is determined ultimately by the rate of hormone synthesis.
1 exception to this rule is the thyroid gland in which thyroglobulin represents storage of the iodothyronines. Thyroid follicles store abundant iodothyronines as thyroglobulin.
Hormone Transport
Water-soluble hormones such as proteins and peptides do not require additional carrier proteins for transport.
Lipid soluble - need transport protein to transfer to another cell.
Insoluble hormones such as iodothyronine and steroids require carrier proteins
Cortisol circulates in the blood in an unbound form (free) or bound to a specific corticosteroid—binding globulin (CBG, transcortin) or to plasma albumin
Corticosteroid-binding globulin is glycoprotein with molecular weight of 51,700 daltons
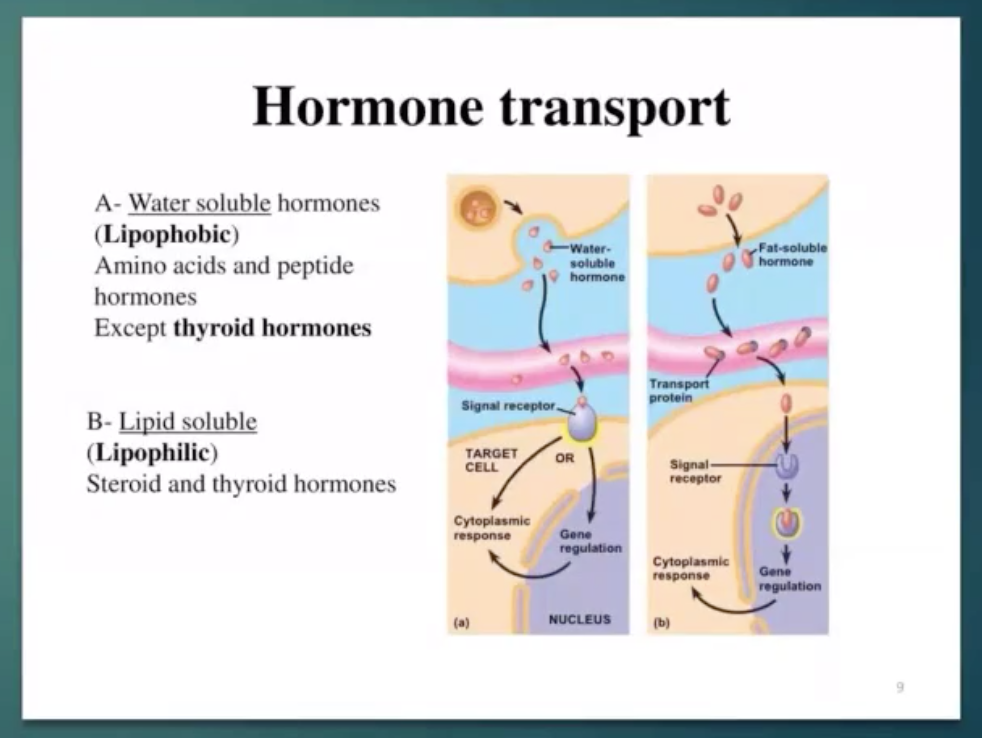
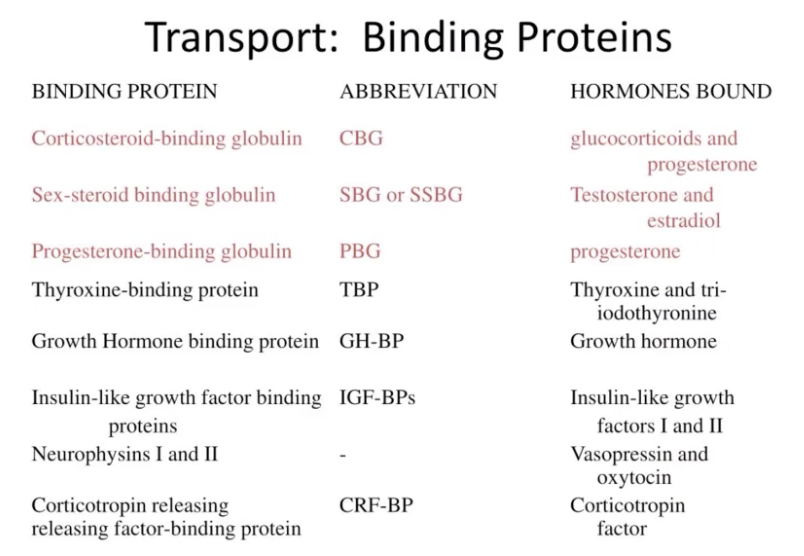 (not sure if sila magkasunod sa slide ni doc hehe)
(not sure if sila magkasunod sa slide ni doc hehe)
Thyroxine binding globulin (TBG) - specific carrier molecule for thyroxine and triodo thyronine. TBG level is increased in pregnancy; but decreased in nephrotic syndrome.
Retinol binding protein (RBP) - it carries vitamin A.
Transcortin / cortisol binding globulin (CBG) - transports cortisol and corticosterone.
Transferrin - carries iron in plasma
Progesterone, prednisolone (synthetic steroid used therapeutically) and aldosterone also bind w/ CBG.
Which results to short acting and long acting steroid.
Carrier protein have significant effects on the clearance rates of hormones.
Upon secretion of T4 and T3, both become reversibly bound to severa liver-derived proteins (like albumin and proalbumin)
Less than 1% of iodothryonines are normally circulating in blood in unbound form.
Total concentration of bound and unbound T3 and T4 in blood not only depend on secretion but also depend on concentrations of their binding proteins in plasma because unbound fractions are vulnerable to metabolism and excretion.
In humans, iodothyronines are bound with high affinity primarily to thyronine-binding globulin (TBG) and plasma half life of T4 is about 7 days.
In dogs, only about 15% of TBG as humans and cats have none.
Dogs & cats rely on low affinity carrier protein such as albumin and prealbumin to bind T3 & T4. Only halg life of T4 in dogs is shortened at 10-16 hours.
Transport of hormones in blood from the endocrine gland to target tissues is not without its problems. Deficiencies of carrier proteins have been documented.
These deficiencies rarely lead to ds because feedback mechanisms compensate adequately.
Transport of Hormones
Hydrophilic hormones
peptides, catecholamines
dissolved in plasma
Hydrophobic hormones
steroids, thyroid hormones
bound to carrier proteins
only free hormone can bind to receptor
only free hormone can be metabolized
longer half-life
Transport of thyroid hormone
T3/T4 are highly bound to plasma protein carrier
Major carrier of thyroxine-binding globulin (TBG)
Secondary carrier are albumin and thyroxine-binding prealbumin
Approx 99.9% of T4 bound and <0.1% is free hormone
T3 free in plasma <1% more active than T4 — bc of tight binging to plasma proteins has long life. T4 last for 7 days.
Free hormone is what captured by target cells
Exerts its biological effect before degradation
Feedback Control of Hormone Production
Feedback control - regulation of hormonal secretion from the endocrine gland by an effect of the circulating hormone that the gland itself produces.
This effect may be direct or mediated by another hormone, a metabolite produced by hormone’s action/physical factor.
Positive Feedback - more hormone
Negative Feedback - less hormone
Ex. Hypothalamus-pituitary-thyroid axis.
In euthyroid (normally functioning thyroid gland) animal thyrotropin releasing hormone (TRH) from the hypothalamus and TSH from the pituitary gland are secreted in the state of equilibrium.
When iodothyronine concentrations in the blood are inadequate
ex. primary hypothyroidism — TRH secretion from the hypothalamus increases to stimulate TSH secretion from the pituitary and TSH stimulates thyroid gland to produce more T4 & T3.
Increased blood concentrations of iodothyronines feedback negatively on the hypothalamus and pituitary gland so that secretions of TRH and TSH are reduced.
Secretions of TSH is controlled at the level of adenohypophysial thyrotrope (TSH-secreting cell) by complex association:
Hypothalamic TRH stimulates synthesis and secretion of TSH
TSH stimulates secretion of iodothyronines by the thyroid gland
T3 interacting locally w/ nuclear receptors decreases TSH synthesis and secretion from the pituitary
Some hormones are under feedback control by the metabolites, physical factors and other hormones.
Ionized Ca negatively controls PTH secretion
Glucose negatively controls glucagon & positively controls insulin secretion.
Volume of ECF negatively controls aldosterone production by feedback mechanisms.
Late in estrous cycle, increasing concentration of ovarian steroid estradiol exert positive feedback influence on the pituitary gland to cause an increase in frequency and amplitude of pulsatile release of LH.
Estrous cycle/Reproductive cycle/Sexual maturity
Ovary > secretes estradiol > hypothalamus release GnRH > anterior pituitar gland release FSH & LH (gonadotrophin)
LH - targets ovary > mature ova to be released & responsible for ovulation.
Increased LH concentrations lead to further increase in estradiol secretion that leads to ovulatory surge of LH.
Few hormones are produced w/o feedback regulation. Those produced by the placenta (ex. equine chorionic gonadotropins or PMSG, progesterone & estrogen) appear not to be regulated by classic feedback mechanisms.
Hormones produced by ectopic glands are free of feedback control.
ectopic pregnancy
Patterns of Hormones in Blood
Concentrations of some hormones in blood change significantly by minute or at least several time within an hour.
Ex. Pulsatile Secretion and release of testosterone whereby each secretory pulse of testosterone is preceded by pulse of LH.
Secretion of glucocorticoids from adrenal cortex is pulsatile rather than at constant rate.
Hormones are not secreted at a uniform rate
Pulsatile pattern - Diurnal/Circadian rhythm - linked to sleep-wake cycles (Cortisol, GH)
Circadian - 24 hr period
Cortisol - high in waking up in the morning
Melatonin - sleep hormone
(When taking melatonin, turn off lights bc the hypothalamus will think it is still morning.)
Be aware of pulsatile nature and rhythmic pattern of hormone secretion when relating the serum hormone measurements to normal values.
In horses, secretory pulses of ACTH in pituitary venous blood generally lasts 10 minutes or less and occur every 15-25 minutes.
Circadian secretory patterns lasts approximately 24 hrs.
In mares, highest cortisol in serum/plasma occur between 7-9AM daily and lowest concentration occur from 7-9PM. Similar patterns have been described in other animals.
Longer intervals between dramatic changes in hormone concentrations are associated w/ estrous cycle.
Changes in progesterone, estrogen & gonadotropin concentrations in blood vary w/ the length of estrous cycle. These intervals are extended by pregnancy & pseudopregnancy.
Thyroxine concentrations in blood also change seasonally, responding to changes in ambient temperature.
General Principles of Hormone Action
The existence of hormone receptors on target cells allows for an understanding of hormone specificity and selectivity of response.
With external / cell membrane receptors and internal / cytosolic receptors, target cells can attract preferentially & bind to exact hormones they need for normal function.
All hormone receptors are proteins and have 2 functional domains:
Recognition domain - one that binds hormone
Coupling domain - one that regulate post-binding biochemical events
The goodness/tightness of binding of the hormone to the recognition domain is measured as the affinity for binding the hormone/K-value.
Perfect fitting hormone has K values of 1. Other molecules have K values from 0-1.
In general, receptors for neurotransmitters, peptides and proteins are located within the cell membrane. Receptors for steroids, vitamin D & iodothyronines reside within the nucleus.
Intracellular Action of Steroid Hormones
Being lipophilic, steroid hormones (like estrogens, progestagens, androgens & glucocorticoids can diffuse across the plasma membrane where they bind to intracellular receptors.
Interaction causes activation of the receptor with resultant changes in receptor conformation.
Steroid-receptor complex then binds to specific region on the cells DNA to promote the transcriptional process w/ the help from protein acceptor molecule.
Intracellular Action of Iodothyronines
Intracellular saturable binders of T3 have been identified in nuclei, mitochondria & membranes of many animal tissues.
Free T3 enters the cell & interacts w/ nuclear receptors
Nuclear receptors is a protein associated w/ DNA composed of single polypeptide chain w/ molecular weight of 50,000-70,000 daltons.
The exact blochemical interaction of the T3-receptor complex with the DNA involves modification of transcriptional events in the nucleus resulting in an increase in specific messenger RNA (mRNA) that codes for the synthesis of specific structural and enzymatic proteins regulated by lodothyronines
These proteins are transported elsewhere to regulate other tissues
They maybe soluble enzymes that regulate metabolic function of the target cell.
They maybe membrane-associated proteins, such as receptors for other hormones.
Intracellular Action of Protein & Polypeptides
Proteins are lipophobic, and do not pass readily through the lipid-rich plasma membrane as do steroids and lodothyronines.
They have receptors in the plasma membrane and rely on Intracellular messengers to transmit signals (signal transduction)to modify cellular function.
Quantification of Biological Fluids
Modern method of hormone quantification for diagnosis of endocrine disease, research, and therapeutic monitoring began with the discovery and development of the radioimmunoassay.
The radioimmunoassay (RIA) is a type of in vitro (in an artificial environment) competitive protein binding assay in which radioactively labeled and nonradio labeled ligand, e.g. hormone, compete for a limited number of binding sites on a binding protein.
Radioimmunoassay - Radioimmunoassay (RIA) is a very sensitive in vitro assay technique used to measure concentrations of antigens (for example, hormone levels in the blood by use of antibodies.
RIA have several advantages over other quantitative procedures for hormones
small sample volumes are needed.
one can assay many samples at a time
little if any sample preparations is required.
RIA can provide excellent specificity, accuracy, sensitivity, and precision.
In addition, most reagents are fairly stable, and there is rapid turnaround of results.
The 4 criteria for assay validity are:
specificity - freedom from interference by substances other than the one intended to be measured.
accuracy - is the extent to which a set of measurements of a substance agrees with the exact amount of the substance that is present.
precision - is the extent to which a given set of measurements of the same sample agrees with the mean.
sensitivity - is defined as the smallest amount of unlabeled hormone that can be distinguished from having no hormone in the sample.
If an endocrine gland fails to develop properly,is destroyed by disease,synthesized a biochemically defective hormone,or is damaged by therapy(iatrogenic), primary hypofunction results.
Primary hypothyroidism is often the results of an autoimmune process whereby the thyroid gland is invaded by immune cells and the hormone-secreting cells are destroyed.
Secondary hypothyroidism - can be due to insufficient secretion of
TSHHypothyroxemia - can be produced by concurrent disease like hyperadrenocorticism,malnutrition and certain drugs
Symptoms Of Hypothyroidism In Dogs
Lethargy
Weight gain
Obesity
Hair loss/coat changes
Low serum thyroid hormone concentrations
Primary hypofunction of the endocrine glands
Results from:
if an endocrine glands tails to develop properly
is destroyed by disease
synthesizes a biochemically defective hormone
is damaged by therapy (iatrogenic)
Primary hypothyroidism - results of an autoimmune process whereby the thyroid gland is invaded by immune cells and the hormone-secreting cells are destroyed.
Secondary hypothyroidism - due destroyed
Hypothyroxemia - presence of an abnormally low level of thyroxine in the blood- can be produced by concurrent disease e.g.
hyperadrenocorticism
malnutrition
certain drugs
Panhypopituitarism in dogs - occur as a result of a developmental defect whereby hormone-secreting cells of the anterior pituitary gland fail to differentiate completely leading to multiple deficiencies such as:
pituitary dwarfism due to lack of growth hormone secretion
secondary
hypoadrenocorticism- reduced
ACTH secretionhypogonadism - diminished FSH and LH secretion
Hyperfunction of the endocrine glands
hyperadrenocorticism - caused by excessive production of cortisol by the adrenal contex
A primary form (primary hyperadrenocorticism) - an adenoma or carcinoma of the adrenal cortex is formed producing cortisol that is not controlled by ACTH
B.secondary hyperadrenocorticism- (Cushing's disease)results from excessive secretion of ACTH by the morphological and functional hyperplasia of the adrenal cortex.
Primary hyperparathyroidism - excessive autonomous secretion of parathyroid hormone
Hyperinsulinemia - in dogs and ferrets - caused by neoplasia of the pancreatic beta cells leading to hypoglycemia
latrogenic endocrine diseases
Caused by unfavorable response to therapy caused by the therapeutic effort itself.
Common iatrogenic endocrine disease is caused by inappropriate or excessive treatment of animals with glucocorticoids.
SIGNS AND SYMPTOMS:
Weight loss
Increased appetite, urination, thirst, respiration
Heart murmurs
Thickened nails
Hyperactivity
Less than 10% of cats with hyperthyroidism are labeled as apathetic
The pituitary gland is subdivided anatomically into:
Adenohypophysis
neurohypophysis
The Adenohypophysis
Has 3 parts:
pars distalis - largest part & contains 5 populations of cels namely:
thyrotropes
gonadotropes
lactotropes
corticotropes
These cells secrete the "Tropic" hormones that regulate other endocrine glands
The tropic hormones are:
TSH - thyrofropin ar thyroid stimukating hormone
LH -Luteinizing hormone
FSH-follicle stimulating hormone
Prolactin
ACTH - adrenocorticotropin
GH - growth hormone or somatotropin (STH)
somatotropes - regulates other non endocrine organs and tissues.
pars tuberalis - upward extension of the adenohypophysis,attached to the infundibulum
pars intermedia - forms the junction between the pars distalis and pars nervosa.
Source of melanocyte - stimulating hormone (MSH) - particularly important in amphibians in which MSH regulates skin pigmentation.
In Cattle pig and rats - ACTH produced by the pars intermedia is cleaved into a-MSH and corticotropin-like intermediate lobe peptide or CLIP
Pars intermedia of dogs and horse - significant source of ACTH.
The Neurohypophysis consists of two parts :
infundibulum or pituitary stalk
pars nervosa also called posterior or neural lobe)
Hormones produced in the hypothalamus and stored in and released from the neurohypophysis are nonapeptides, these includes the following :
oxytocin
arginine vasopressin
lysine vasopressin - present in pigs
Arginine vasotocin
Neurohypophysis possess a rich supply of nerves.
Fibers originating from the paraventricular,supraoptic and other hypothalamic nuclei enter the neurohypophysis via the
infundibulum.These fibers contain oxytocin,vasopressin, or vasotocin in nonmammalian species.
Fibers contain their respective carrier proteins called neurophysin
Hypothalamic Control of the Pituitary Gland
Every hormone produced by the adenohypophysis is regulated by at least one hormone synthesized in hypothalamic nuclei and released into the blood of the hypophyseal portal system to be transported to the pars distalis.
Hypothalamic hypophysiotropic substances that stimulates pituitary function originally were called RELEASING FACTORS after the initial designation of corticotropin-releasing factor (CRF)
Now,CRF and other releasing hormones are considered true hormones,secreted for hemocrine communication.
All hypophysiotropic hormones except dopamine (prolactin-release inhibiting hormone) are peptides.
The synthetic stimulatory hypophysiotropic hormones are:
TRH - thyrotropin - releasing hormone
GRH - gonadotropin -releasing hormone
CRH - corticotropin -releasing hormone
GHRH - growth hormone-releasing hormone
Originally believed that pituitary hormones were regulated ONLY by stimulatory hypothalamic factors.
Now, some hormones are under control by inhibiting hormones secreted by the hypothalamus.
Prolactin, growth hormone & TSH are regulated by both releasing and inhibiting hormone.
Actions of individual hypophysiotropic hormones are not limited to a single pituitary hormones. (e.g TRH not only stimulates release of TSH but also induces the release of prolactin & GH, GnRH stimulates the release of both LH & FSH.)
They also regulate pituitary cell differentiation, proliferation & hormone synthesis.
Hormones secreted by the Anterior Pituitary
Gland
FSH & LH > Testes & ovaries
TSH > Thyroid
ACTH > Adrenal cortex
Prolactin > Mammary glands
MSH > Melanocytes
GH > Liver, bones, other tissues
Parts of Posterior Pituitary Gland
Pars nervosa - contains capillaries, small neuroglial cells called pituicytes and non myelinated axons that extends to the pars nervosa from neurons in the supraoptic and paraventricular nuclei of the hypothalamus.
These axonal fibers contains secretory granules of neurohypophyseal hormones:
oxytocin
arginine vasopressin - also called antidiuretic hormone or ADH.
Oxytocin and AVP are both nonapeptides with sulfhydryl bond between two cysteine residues at positions 1 and 6.
Pigs and other members of the suborder Suina produce lysine vasopressin which contains lysine instead of arginine in position 8.
Oxytocin and AVP are released into the capillary blood in the pars nervosa.
Cell bodies of the neurons producing AVP and oxytocin are located in the paraventricular and supraoptic nuclei at the base of the hypothalamus.
Cells of the hypothalamic nuclei initially synthesized prohormones.
In the Golgi apparatus the proteins are glycosylated and packaged as neurosecretory granules.
The prohormone-containing granules are transported down the neuronal axons to their terminals in the pars nervosa.
During transport,these prohormones are cleaved to yield AVP or oxytocin and their binding proteins called neurophysin.
The binding protein for oxytocin is Neurophysin 1 and for AVP is Neurophysin 11.
The hormone neurophysin complex stabilizes the hormone within the neurosecretory granules.
Release of hormones & neurophysin from neurosecretory granules is initiated by electrical signals from sensory receptors monitoring osmolarity of ECF.
Action potentials generated in the osmoreceptors cause an influx of calcium into the axonal terminals and AVP is released by exocytosis.
Upon released into the blood AVP and Neurophysin dissociate from each other.
Hydration of the body - injection of saline solution into the blood going to the hypothalamus INHIBITS release of AVP leading to resorption of less water from the glomerular filtrate.
Excess water is excreted from the body as diluted urine.
Dehydration or injection of hypertonic electrolyte solutions into the hypothalamus stimulates release of AVP causing increased water resorption in the distal tubules and decreased glomerular filtration resulting in less urine being produced.
The major effect of AVP is to increase reabsorption of water from the glomerular filtrate.
Oxytocin is stored as neurosecretory granules and is released from axonal terminals by calcium-dependent exocytosis.
The primary stimuli for oxytocin release form the storage sites in the neurohypophysis are:
distention of the reproductive tract particularly in the pregnant female
stimulation of the mammary gland by the young
audio visual contact with the offspring
Oxytocin has specific effects on the contraction of smooth muscle of the uterus and cells of the mammary gland.
In Veterinary Medicine oxytocin is:
used for inducing parturition in some species
increase uterine contractions at parturition
for the treatment of retained placenta, me tritis and agalactia.
Oxytocin is stored as neurosecretory granules and is released from axonal terminals by calcium-dependent exocytosis.
The primary stimuli for oxytocin release form the storage sites in the neurohypophysis are:
distention of the reproductive tract particularly in the pregnant female
stimulation of the mammary gland by the young
audio visual contact with the offspring
Oxytocin has specific effects on the contraction of smooth muscle of the uterus and cells of the mammary gland.
In Veterinary Medicine oxytocin is:
used for inducing parturition in some species
increase uterine contractions at parturition
for the treatment of retained placenta,metritis and agalactia.
Disorders of the Pituitary Function
The anterior pituitary secretes six major hormones- any deficiency of these can occur such as:
secondary hypothyroldism
secondary hypoadrenocorticism
Panhypopliuitarism - secretion of all hormones from the anterior pituitary is abnormally low or absent
CLASSIC ENDOCRINE GLANDS
pituitary glands
thyroid glands
parathyroid glands
endocrine pancreas
adrenal glands
gonads (a.ovaries & b.testes)
MORE RECENTLY IDENTIFIED ENDOCRINE GLAND
kidneys
heart/blood
liver
fat(adipose tissue)
placenta
Adrenocorticotropin is a 39-amino acid peptide derived from
proopiomelanocortin(POMC,MW 28,500 daltons)
Other peptides with hormonal activity derived from POMC include:
B-endorphin
B-lipotropin
a-melanocyte-stimulating hormone (a-MSH)
Secretion of ACTH is regulated by hypothalamic CRH and AVP
Arginine vasopressin is a weak regulator of ACTH > it acts synergistically with CRH to stimulate secretion of ACTH
After binding to its receptor on corticotropes, i.e,pituitary ACTH secreting cells, there is an accumulation of cyclic AMP that serves as the second messenger.
CRH stimulates the release of ACTH in a pulsatile manner
Circadian rhythm with the highest pulse frequency of ACTH release in blood occurs just before and during the hour of awakening in the morning.
Followed by a progressive decline in ACTH release throughout the remaining of the day.
Studies to examine pulsatile circadian secretion of ACTH require frequent collection of blood samples for analysis of hormone concentration
These must be done with great care because hemorrhage, even minor, is a potent stimulus for the secretion of ACTH and cortisol.
Handling of animals during blood sampling causes significant elevations in serum cortisol concentrations, even after previous adaptation.
Other internal and external stress stimuli increase ACTH and cortisol secretion in prenatal and post natal domestic animals.
hypoxemia
hypotension
hypoglycemia
ambient temperature
surgery
trauma
pain
ACT stimulates the cortex of the adrenal gland to secrete the steroid hormone cortisol in most mammals or corticosterone in rodents and lagomorphs.
ACTH acts on the two inner zones (zona fasciculata and reticularis) of the adrenal gland to increase cortisol or corticosterone secretion.
Adrenal cortex responds to ACTH morphologically by hypertrophy of cells in the zonde fasciculata and reticularis, functionally by increased production of glucocorticoids.
Synthesis of adrenocortical steroids requires:
cleavage of the side chain from the 27-carbon cholesterol to form a 21-carbon steroid called pregnenolone
various hydroxylations of pregnenolone
oxidation of the 3B-hydroxyl to aketone
shift of the double band from carbons 5 and 6 to carbon 4 and 5.
Four major sources of cholesterol are available to the cell to meet its metabolic needs:
hydrolysis of intra -cellular cholesterol ester
de novo cholesterol
direct delivery into the cell by passive diffusion of mononuclear cholesterol
up take of cholesterol associated with plasma lipoproteins
The following are stimulated by ACTH:
up-take of lipoproteins by adrenal cells
conversion of a cholesterol ester to cholesterol
side-chain cleavage of cholesterol
Intracellular biochemical events stimulated by hormones involve:
activation of adenylate cyclase
accumulation of cyclic AMP
increased protein kinase activity
phosphorylation of regulatory proteins
Prolactin - lactotropin or mammotropin
Single chain polypeptide hormone
MW -23,000 daltons
Produced by lactotropes or mammotropes
Named because of the indispensable role in lactation.
Prevalent in all vertebrates from fish to humans
Major roles: both reproductive and nonreproductive events
regulation of metamorphosis in amphibians
osmoregulation in teleost fish
proliferative effects on male accessory organs
regulation of parental behavior in several species.
stimulation of the brood patch in the sparrows
stimulation of crop milk formation in pigeons
important in maintaining structure and function of the corpora lutea of female rodents.
stimulates development of receptors for LH on Leydig cells,hence prolactin indirectly stimulates secretion of testosterone
Prolactin is also called luteotropic hormone (LTH)
Role in differentiation and maintenance of the mammary gland and secretion of milk are of primary importance.
Stimulus for the let down of milk is suckling
Neurogenic impulses are carried to the brain and to secretory
neurons in the hypothalamus that
release hypophysiotropic hormonesHypophysiotropic hormone the hypophysed portal system.
In Pituitary gland prolactin secretion is stimulated or inhibited by this hormone
Prolactin is under inhibitory control by dopamine,secreted by
the hypothalamus
Prolactin is released in a pulsatile manner.
Pituitary lactotropes require constant inhibition by dopamine to keep prolactin secretion under control.
Other prolactin inhibiting factors:
y (gamma) aminobutyric acid
GnRH-associated peptide
Naturally occurring compounds that release prolactin:
TRH
VIP / Vasoactive intestinal peptide
Serotonin
B endorphin
Somatostatin
Gastrin
GnRH
Vasopressin
Oxytocin
Angiotensinogen 2
Thyrotropin-releasing hormone and VIP are prolactin releasing hormones.
Both are directly secreted into the hypophyseal portal blood directly stimulate prolactin release from the pituitary gland.
VIP is synthesized by the adenohypophysis and is present in lactotropes, thus VIP is a hemocrine and autocrine regulator of prolactin secretion.
Prolactin is synthesized processed.packaged, stored and released by lactotropes.
Dopamine TRH, and VIP affects both synthesis and release of prolactin
Receptors for these three are membrane bound.
Second messengers:
cAMP
ionized calcium
phospholinositides
Primary mechanism of dopamine action may be inhibition of cAMP production.
Vasoactive intestinal peptides stimulates adenylate cyclase activity and production of cAMP
Calcium ,phospolinositides,lesser extent. CAMP may be involved in the release of prolactin.
Physiological actions of prolactin are mediated through specific cell-surface receptors in cell of mammary gland, liver, ovary, testes, and prostate.
Intracellular second messengers for prolactin include:
ionized calcium
polyamines (derivatives of the amino acid arginine)
prostaglandins
The main site of action is the mammary gland
During pregnancy the following hormones play roles in the development of the milk secretory apparatus:
insulin
cortisol
triodothyronines(T3)
estrogen
progesterone
growth hormone
prolactin
Estrogen and progesterone inhibit lactation during pregnancy.
After parturition, estrogen and progesterone concentration in the blood decrease rapidly, allowing prolactin to initiate lactation.
Effects of prolactin on lactation
Includes:
regulation of transcription of genes that encode for milk proteins ,such as casein
stimulation of epithelial cell proliferation in mamma
Growth Hormone / Somatotropin / HGH
Growth hormone or Somatotropin- single chain,nonglycosylated protein
Molecular weight - 22,000 daltons secreted by the pituitary gland
Its pulsatile secretion is regulated by:
hypothalamic growth hormone-releasing hormone (GHRH)
growth hormone release inhibiting factor or Somatostatin (SRIF)
GHRH controls growth hormone synthesis and release
Intracellular second messenger is cyclic AMP
The following are intracellular mediators:
ionized calcium
diacyglycerol
inositol triphosphate
Somatostatin reduce GH secretion independently of GHRH & by blocking GHRH action.
Growth hormone has major actions involving metabolism, growth and cellular differentiation.
Growth hormone:
increases lipolysis in adipose cells
glycogenolysis and protein synthesis in liver and muscle cells
chondrogenesis in bone
it interacts with membrane receptors of the liver to cause the release of growth-stimulatory peptides called somatomedins.
Somatomedins are single chain proteins that resemble proinsulin.
They circulate in blood complexed with binding protein
Other growth promoters that are important participants in tissue growth and organ development:
1 insulin-like growth factor 1 (IGF-1)- previously known as Somatomedin C important mediator of growth hormone action, produced by the liver. It also feeds back on the pituitary gland to regulate hormone secretion
insulin-like growth factor 2 (IGF-2)-secreted by cells of the CNS and is involved with fetal tissue development
Blood concentration of IGF-1 are low in growth hormone deficiency i.e. dwarfism high in growth hormone excess. i.e. acromegaly Plasma concentration of GH and IGF-1 are correlated with body size.
Standard poodles had six times the mean plasma concentration of IGF-1 as Toy Poodles
GH plays a central role in lactation and increases both the soft and osseous tissue masses of the body.
Concentrations of GH in blood are high during rapid growth in cattle,swine and poultry.
Growth of the long bones continues as long as the epiphyseal plates do not close
In domestic animals closure of the epiphyseal plates after puberty result in the cessation of skeletal growth under normal condition.
Prolonged administration of growth hormone to dogs and several other species can induce permanent hyperglycemia therefore GH is diabetogenic.
High blood glucose concentrations stimulate beta cells of the pancreatic islets to produce insulin until they are eventually exhausted and undergo degeneration.
Growth hormone injected into growing and lactating animals leads to improved nutrient utilization.
Pharmacologic doses of GH injected to growing pigs and lambs:
increase nitrogen retention
improve feed efficiency
increase muscle mass
reduce carcass lipid content
increase carcass protein content
In high-producing dairy cows,GH such as genetically engineered bovine somatotropin (BST) increases milk production 10 to 15% without affecting feed intake.
Enhances the ability of the mammary tissue to synthesize milk components.
Pituitary Glycoprotein Hormones - TSH, LH & FSH
Consist of two noncovalently bound polypeptide chains called alpha and beta subunits.
These polypeptide hormones consist of sugar constituent including:
D-mannose
D-galactose
L-fucose
D-glucosamine
D-galactosamine
Sialic Acid
Thyrotropin or Thyroid-Stimulating Hormone (TSH)
Have only one physiological function -stimulation of the thyroid gland
Nearly all steps in the synthesis and secretion of thyroxine(T4) and 3,5.3trilodothyronine are enhanced by TSH
The primary regulator of TSH secretion is feedback by T3 on the pituitary gland to inhibit TSH synthesis and on the hypothalamus to inhibit TRH synthesis.
Lactotropes and thyrotropes of the pituitary gland contain receptors for TRH.
Hence, TRH simulates the in vivo secretion of prolactin and secretion of TSH.
Gonadotropins
A.LH and FSH are glycoprotein consisting of alpha and beta subunits associated by noncovalent bonds.
The alpha is common to both hormones,specific hormone activity is associated with the beta subunits
Combination of both subunits is required for biologic activity
Secretion of gonadotropin is regulated by:
gonadal steroids
ex. estrogens, androgens,progesterone
Inhibin - a glycoprotein with two polypeptide subunits synthesized by :
A. sertoli cells of the testes
B. granulosa cells of the ovary
C. placenta
D. pituitary gonadotropes
E. brain
Inhibin feedback negatively on the hypothalamus and pituitary gland to specifically reduce secretion of FSH
Gonadotropes have specific membrane receptors for GRH
Gonadotropins are bihormonal. They synthesize both LH and FSH.
LH in male animals is referred to as Interstitial Cell-Stimulating Hormone (ICSH)
Disorders of the Pituitary Function
The anterior pituitary secretes six major hormones- any deficiency of these can occur such as:
secondary hypothyroidism
secondary hypoadrenocorticism
Panhypopituitarism - secretion of all hormones from the anterior pituitary is abnormally low or absent
Clinical manifestations - associated with diminished growth secretion and dwarfism.
Juvenile panhypopituitarism occurs more frequently in GS dogs.
Basal growth hormone and IGF-1 concentrations in plasma of dwarf dogs are greatly reduced.
Growth hormone concentrations do not increase after injection of clonidine -a standard dynamic test for growth homone secretory capacity.
Affected pups appear normal at birth, usually indistinguishable from littermates up to about 2 months of age.
Indicative of dwarfism:
slower growth rate relative to their littermates
retention of puppy hair coat
lack of primary guard hairs (coarse hair covering the underfur)
Acromegaly is a disease caused by excess growth hormone secretion.
Clinical manifestations:
overgrowth of connective tissue
increased growth of bone
coarsening of facial features
enlargement of viscera
Most common cause of acromegaly in cats - tumors of somatotropes
Most common cause of acromegaly in dogs-due to somatotropic hyperplasia induced by progesterone-like compounds
Diabetes insipidus - is a disorder characterized by chronic excretion of large volumes of dilute urine, accompanied by extreme thirst caused by hyperosmolarity of body fluids and dehydration.
Central diabetes insipidus - is caused by inadequate production of AVP by the posterior pituitary gland.
Central diabetes insipidus - results mainly from the destruction of the supraoptic and paraventricular nuclei of the hypothalamus, where AVP is produced.
Can be caused also by the destruction of the axons carrying AVP to axonal terminals in the pars nervosa.
Nephrogenic diabetes insipidus - produced by several disorders that interfere with the interaction between AVP and its receptors in target cells of the kidney.
The lesions responsible for the disruption of AVP secretion include:
large pituitary neoplasms
cyst
inflammatory granuloma
traumatic injury to the skull
Pituitary - like Hormones of the Placenta
Most of the placental protein and peptide hormones are structurally and functionally similar hormones produced by the pituitary gland and hypothalamus.
Several placental hormones are useful therapeutic agents for Veterinary Medicine.
placental lactogen - protein hormone so-called because it has lactogenic properties and prolactin-like activity.
Ovine placental lactogen(oPL)- has an amino acid composition similar to ovine prolactin and ovine growth hormone. oPL has been localized in secretory granules of mononucleate and binucleate cells of trophoblastic component of the placenta.
Bovine PL has been localized in the binucleate cells of fetal placenta.
Placental lactogen appear to play roles in:
regulating mammary gland function
fetal growth
maternal intermediary metabolism
ovarian steroidogenesis
After 5th week of pregnancy - concentrations of oPL in the circulation in the ewe increase steadily.
Reach highest concentration between 120 and 140 days of gestation, decline until parturition.
Concentrations are greater in ewes carrying twins or triplets
Gonadotropins
Chorionic (i.e.placental) gonadotropins -used commonly in Veterinary Medicine to duplicate the biological effects of LH and FSH
human Chorionic Gonadotropin(hCG) for medical used is obtained from the urine of pregnant women.
A. It closely mimics the effects of LH and has some FSH activity
In female animals - promotes maturation of ovarian follicles,ovulation,and formation of testosterone
In males :
It stimulates testicular interstitial cells to produce testosterone
used clinically to treat:
ovarian follicular cysts
nymphomania (constant or frequent heat)
cryptorchidism male infertility
to induce or hasten ovulation
B. Used also in dynamic diagnostic tests if remnant testicular tissue is present in castrated male dogs and cats and if remnant of ovarian tissue is present in OVH.
hCG ang its subunits has been localized primarily in syncytio-trophoblast of the placenta.
Placenta of the mare - produces gonadotropin called eCG / equine chorionic gonadotropin or PMSG / pregnant mare serum gonadotropin
It is synthesized by endometrial cups of the uterus, begin to develop about 36 days of pregnancy in mare.
Cups begin to degenerate by day 60 of gestation but persist until about day 120 of pregnancy.
In mares, eCG appears in maternal blood on about day 40 of pregnancy.
Between day 55 and day 65, the blood concentration increase rapidly to peak values decline to very low concentration by day 125.
Equine CG has high FSH activity, administered to cow to induce superovulation for embryo transfer.
Anatomy of the Thyroid Gland
In most animal species, there are 2 thyroid lobes located on the latera surface of the trachea.
In pigs, the main lobe of the thyroid is on the midline in the ventral cervical region with dorso lateral projections from each side.
In dogs, the right lobe of the thyroid is situated cranial to the left lobe and almost touches the causal aspect of the larynx.
Histology of the Thyroid Gland
The thyroid gland contains follicles ranging from 10-250 um in size, filled with colloid produced by the cells lining the follicle.
The follicular cells are cuboidal to columnar and their secretory polarity is directed toward the lumen of the follicle.
Most important functional cells of the thyroid are the follicular cells. Depending on the intensity of the stimulation by the Pituitary TSH, they vary in height, ranging between cuboidal (low TSH) to columnar (high TSH) in appearance.
Synthesis of the Thyroid Gland
Essential raw materials such as Iodide from the plasma are trapped by follicular cells, transported rapidly against concentration gradient to the lumen & oxidized by peroxidase to from Iodine.
The assembly of the thyroid hormone within the follicular lumen is made possible by unique protein called thyroglobulin, which is synthesized by follicular cells.
Foods rich in iodine:
dried seaweed
cod fish
yougurt
baked potato
eggs
tuna
turkey
cranberries
strawberries
Small amount of iodide is lost in the feces (about 20-25 ug per day at the usual levels of intake) possibly via secretion into the colon.
The principal features of iodine metabolism in the dog, for the daily iodide intake slightly in excess of normal requirement are as follows:
Dietary intake of 160 ug is augmented by 65 ug of recycled iodide, approximately 50 ug of this recycled iodide is released by the thyroid glad and 15 ug us derived from the peripheral degradation of T3 & T4.
2/3 of this combined input 225 ug is excreted chiefly in the urine.
Coupling (peroxidase enzyme):
MIT + DIT = T3
2 DIT = T4
T3 and T4 remain stored in the colloid
Other tissues that have the capacity to actively transport iodide at a much lower level than the thyroid:
salivary gland
gastric mucosa
lactating mammary gland
The active transport of Iodide is associated with sodium iodide symporter / NIS
Transport of Iodide ion across the thyroid cell membrane is linked to the transport of Na
Ion gradient generated by the Na K ATPase appears to be the driving force for the co-transport of Iodide.
The transported protein is present in the basolateral membrane of thyroid follicular cells & large protein.
Iodine is bound to tyrosyl residues in thyroglobulin at the apical surface of follicular cells to form MIT / monoiodotyrosine and DIT / diiodotyrosine.
The resulting MIT and DIT combine to form 2 biologically active iodothyronine:
T4 / thyroxine
T3 / triiodothyronine
The storage of thyroglobulin in the follicular lumen is essential for maintaining constant levels of thyroid hormone under conditions of varied intake of iodine and varying demands for T4 and T3
Thyroglobulin
high molecular weight glycoprotein
synthesized on the ribosomes of the ER of follicular cells
The constituent amino acids called Tyrosine & carbohydrates.
ex. mannose, fructose, galactose (which comes from the circulation.)
synthesized thyroglobulin leaves the golgi apparatus and packaged into apical vesicles and extruded into follicular lumen.
Amino acid Tyrosine, essential component of thyroid hormone is incorporated within the molecular structure of thyroglobulin.
 Knowt
Knowt