🧬 AP Biology Unit 3
12-16% AP Weighting
Topics
3.1 - Enzyme Structure
3.2 - Catalysis
3.3 - Environment Impacts on Enzyme Functioning
3.4 - Cellular Energy
3.5 - Photosynthesis
3.6 - Cellular Respiration
3.7 - Fitness
Important Vocabulary or Quick Review
Metabolism - All of an organism's chemical reactions
Catabolic Pathways - Metabolic pathways that release energy by breaking down complex molecules
Anabolic pathways - Metabolic pathways that consume energy to build complex molecules.
Kinetic Energy - The energy of motion
Potential Energy - The energy an object possesses due to its location/structure
Chemical Energy - A form of kinetic energy in molecules due to the arrangement of atoms
Thermodynamics - The study of energy transformations
Open system - A system where things can enter and exit, used in biology
Closed System - A system where things cannot enter or exit.
1st Law of Thermodynamics - Energy cannot be created or destroyed
2nd Law of Thermodynamics - Every energy transformation makes the universe more disordered
Entropy - The measure of disorder
Exergonic Reactions - Reactions that release energy.
Endergonic Reactions - Reactions that absorb energy, nonspontaneous.
Delta G - The energy “cost,” negative when the reaction is exergonic.
Allo - Distant
Positive feedback - a continuous, self-feeding cycle, like the snowball effect
Negative feedback - Fixes what causes it to start, so it ends.
Oxidative reactions - A reaction with the loss of an electron (OIL RIG)
Reduction reactions - Reactions with the gain of an electron.
Stoma - A tiny leaf hole
Xylem - Plant veins
Photosystems - Proteins within the thylakoid membrane that store chlorophyll.
3.1-3.4 - Energy Basics
Click Here for a video on energy
Click Here for a video on enzymes
Click Here for a video on enzymes, metabolism, and thermodynamics
Metabolism is the name for all of an organism's chemical reactions. Metabolic Pathways alter molecules in a series of steps. Catabolic pathways release energy by breaking down molecules. Anabolic pathways consume energy to build more complex molecules. Energy released by catabolic pathways power the anabolic pathways. Cellular respiration is a catabolic pathway and Photosynthesis is an anabolic pathway. Photosynthesis and Cellular respirations are opposites.
Energy is required to do work, such as rearranging matter in molecules. Energy can be converted from one form to another.
Kinetic Energy is the energy of motion
Potential energy is an energy that an object possesses due to its location or structure.
Chemical energy is a form of potential energy in molecules due to the arrangement of atoms having the potential to release energy when broken apart
Thermodynamics is the study of energy transformations. There are closed systems and open systems, biology mainly deals with open systems. Closed systems will reach equilibrium and stop moving energy, and therefore will die.
The First Law of Thermodynamics - Energy cannot be created or destroyed.
The Second Law of Thermodynamics - Every energy transformation makes the universe more disordered. Entropy is the measure of disorder, so Entropy levels increase with every energy transformation
Organisms live off free (often chemical) energy. They take more complex, or more entropic, molecules and turn them into simpler molecules to release and harness free energy. Spontaneous reactions can occur without the need for energy input, (but still require activation energy!) while non spontaneous reactions require an energy input to begin and result in a loss of energy.
An Exergonic Reaction releases free energy and the Delta G (△G) is negative. Delta G is the “energy cost” of a reaction. They are spontaneous.
An Endergonic Reaction absorbs free energy from its environment, so Delta G is positive. It is non-spontaneous.
Cells do 3 main kinds of work that require energy (often ATP)
Mechanical Work
Muscles, cilia, moving chromosomes
Movement using Motor Proteins
Transport Work
Movement of molecules
Active Transport
Chemical Pumps
Chemical Work
This drives endergonic reactions
Breaking down or building of molecules
ATP is a nucleic acid with 3 phosphate groups and a nucleotide. In order to “power” a molecule you phosphorylate it, meaning ATP will give up one of its 3 phosphate groups, which provides energy for a protein to use. It becomes ADP. Humans use ATP to power reactions, which makes molecules more unstable and reactive, (P on someone, they become unstable and reactive, same with molecules)
Enzymes (Catalytic Enzymes) speed up reactions by lowering the activation energy required for the reaction to begin. All reactions, including exergonic reactions, require activation energy. Heat, a form of energy, is required for a cell to be metabolically active, but too much heat can kill the cells by denaturing the vital proteins. So, we use enzymes to lower the amount of heat energy needed for reactions.
Enzymes are substrate specific. (Substrates are the reactants) Substrate(s) will bind to an enzyme and it will “bite down” in an induced fit. More catalytic enzymes can help with a reaction go forward or backward, so they will work towards an equilibrium.
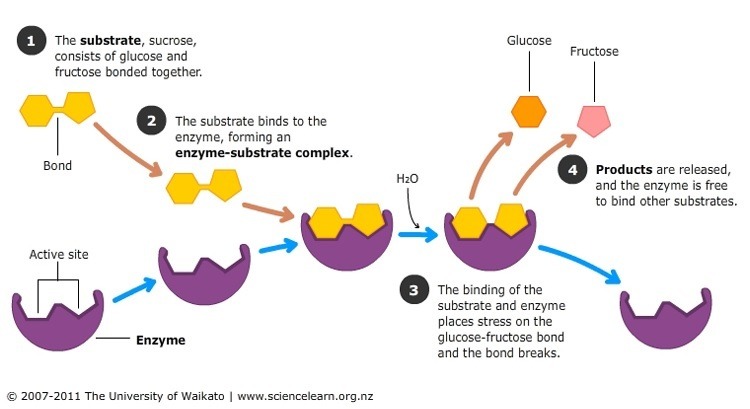
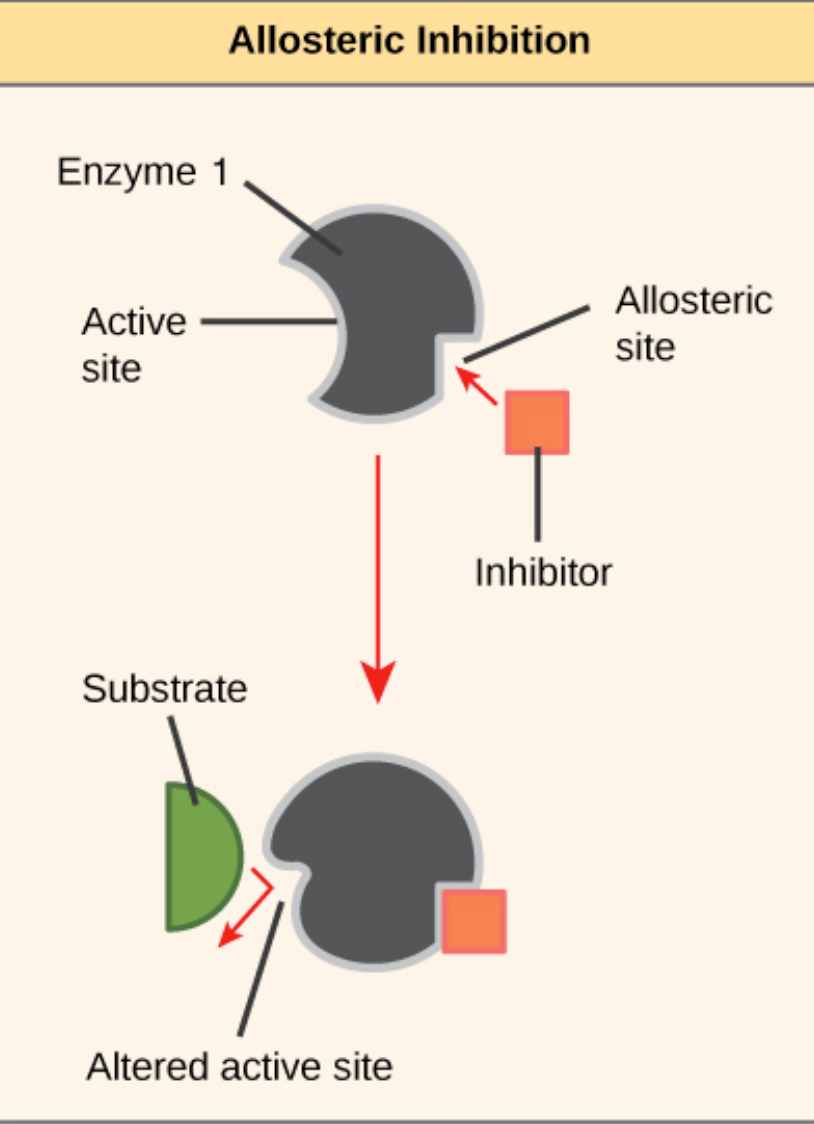
Competitive inhibitors compete with substrates to bind to the active site. If the competitive inhibitor binds first, the substrate cannot bind, and therefore the enzyme does not work. No competitor inhibitors bind somewhere other than the active site, called the allosteric site, to stop it from working. Binding to an allosteric site causes an induced fit, which is too tight for the substrate to get in and bind to.
If a reaction tries to occur in an environment with too much or too little heat, it will either work slowly or not work at all. This also applies to pH, most enzymes prefer a pH of 6-8, however others, like digestive enzymes in stomach acid, work outside of this range. The suffix for most enzymes is -ase.
Electron Carriers
Electron carriers carry electrons. (Big shock!)
Empty -> Carrying electron
NAD+ -> NADH
FADH -> FADH2
NADP+ -> NADPH
Hydrogen works like a cap to a jar (electron carrier) holding an electron.
3.6 Cellular Respiration
Click Here for a video on Cellular Respiration
Click Here for a video on Fermentation
There are three stages to respiration:
Glycolysis
In the Cytosol
Produces 2 ATP
Krebs Cycle
In the Mitochondrial Matrix
Produces 2 ATP
Oxidative Phosphorylation
On the inner membrane
Produce ~30-34 ATP
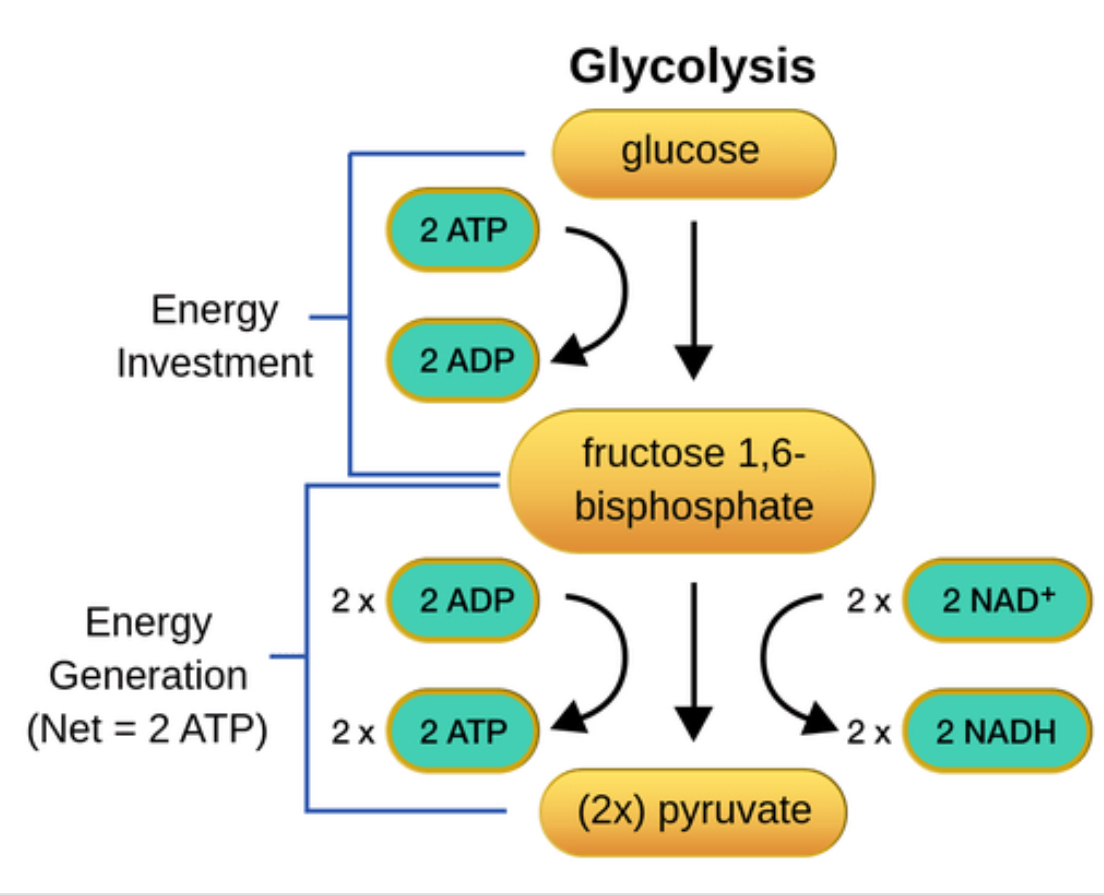
Glycolysis
The first step of glycolysis is called the energy investment phase as it requires an energy input of 2 ATP. 6 carbons are turned into 2 groups of 3 carbon. (Pyruvate) 4 ATP is produced, which means there is a net gain of 2 ATP. This also fills 2 NADH (full electron carriers).
2 ATP + Glucose + 2 NAD+ → 2 NADH, 4 ATP, 2 Pyruvate
or
Glucose + 2 NAD+ → 2 NADH, 2 ATP, 2 Pyruvate
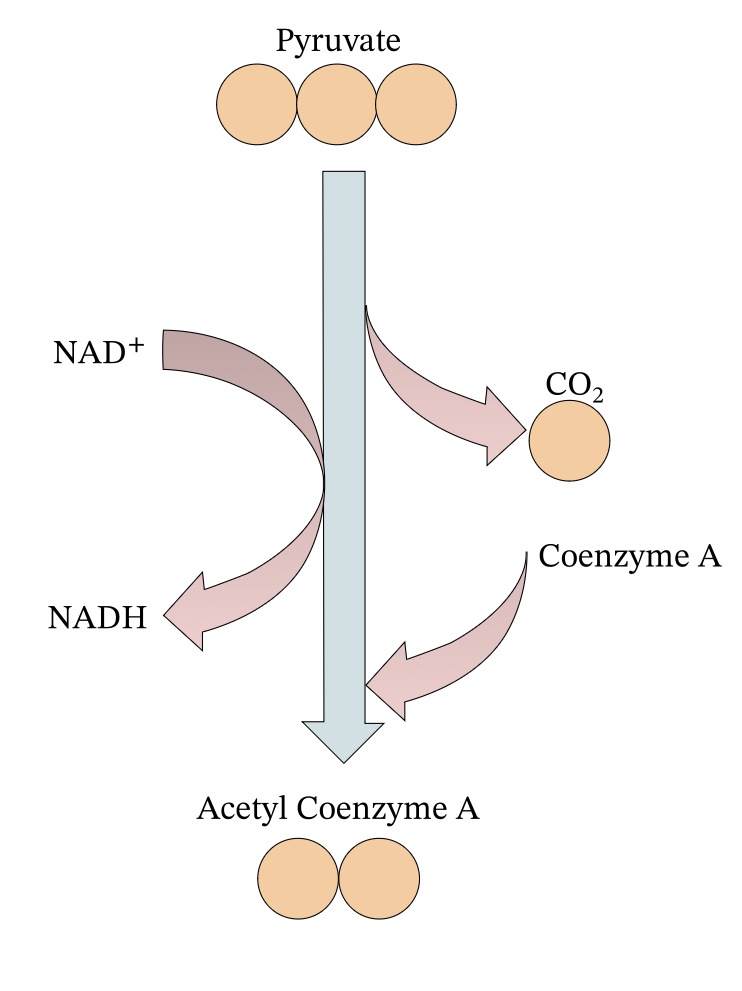
The Intermediate Process
Pyruvate is turned into 2 Acetyl CoA, 2 (waste) CO2, and fills two more NADH.
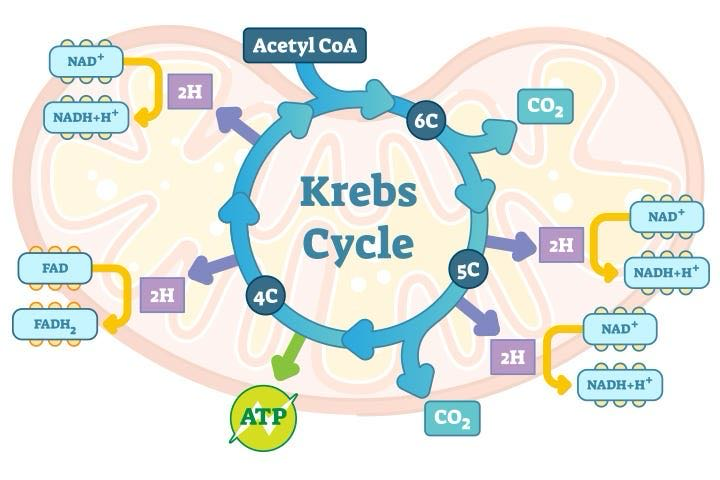
Kreb’s Cycle or Citric Acid Cycle
2 Acetyl CoA is put in and 2 ATP and 8 Electron Carriers (2 FADH2 and 6 NADH) are produced. The photo below happens twice, once for each Acetyl CoA.
Oxidative Phosphorylation
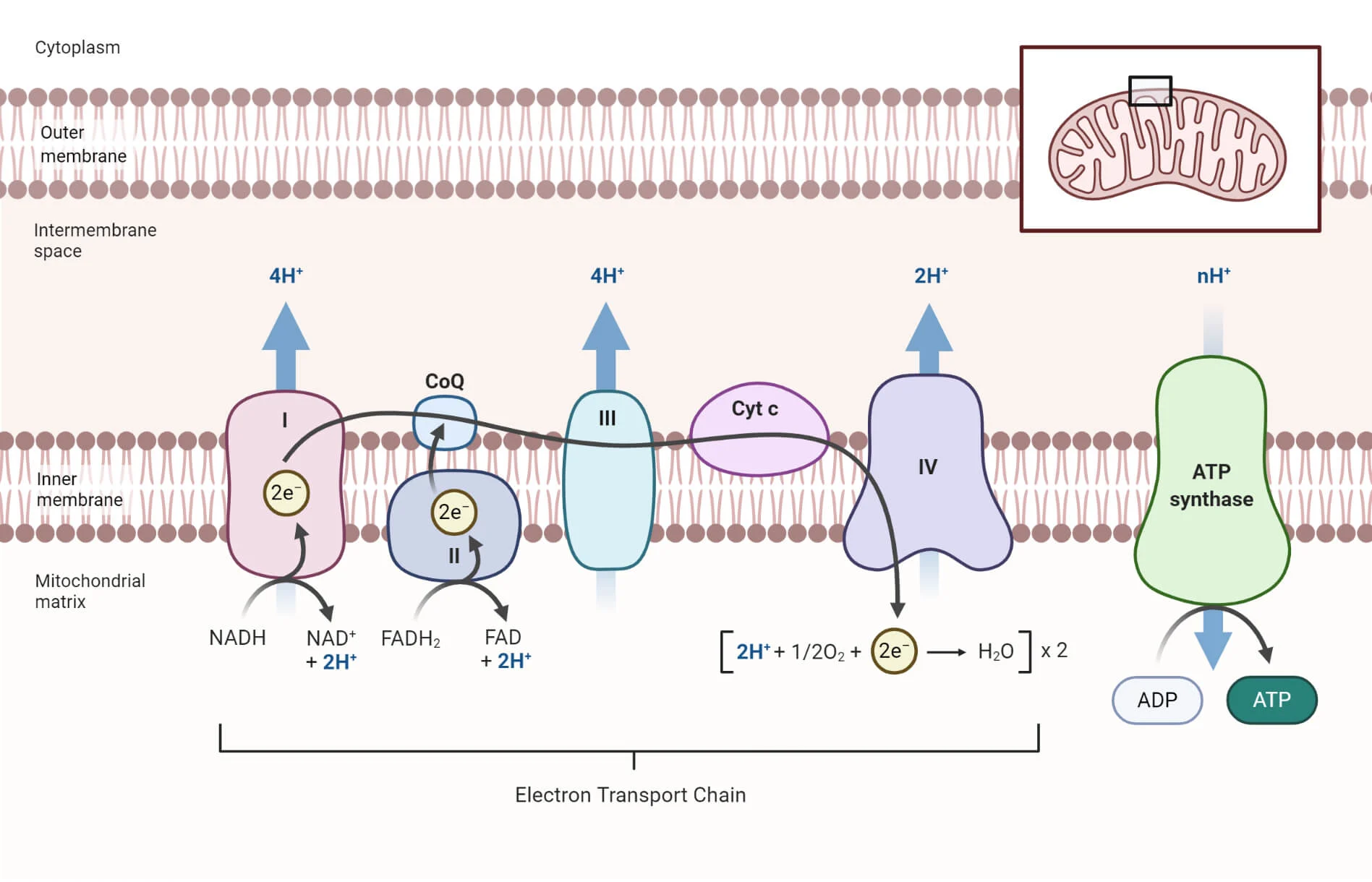
The Electron Transport Chain is a series of proteins that transport protons across the membrane so ATP synthase can produce energy by allowing the protons to return to lower concentrations. The further along the chain, the less energy there is. At the top an electron carrier hands off an electron to the first protein. Each protein oxidizes the protein before it by reducing themselves, aka it takes the electron from the earlier protein. Each time a protein takes an electron from the previous protein, an H+ ion (proton) is pumped from the matrix to the intermembrane space. This pumping is called proton-motive force. At the end, oxygen, the final electron acceptor, binds to the electrons and makes water, a waste product.
ATP Synthase is a protein that synthesizes (makes) ATP through chemiosmosis. It is shaped like an upside down mushroom and goes through the inner membrane. Hydrogen protons flow through it down its concentration gradient from the matrix to the intermembrane space. This causes the protein to spin and push together ADP and P, making ATP. The movement of hydrogen from high to low concentration to generate ATP is called chemiosmosis.
Fermentation
Fermentation is another form of cellular respiration, anaerobic (no oxygen) respiration. It occurs when a cell doesn't have enough oxygen to complete aerobic (oxygen) respiration.
Lactic Acid Fermentation starts off with glycolysis which produces 2 ATP. The NADH is turned into NAD+ by turning Pyruvate into lactic acid, NADH gives the electron to pyruvate. This allows the NAD+ to continue this process by completing glycolysis again and create 2 more ATP.
Alcoholic Fermentation is done by bacteria who don't have a mitochondria. They do glycolysis and then turn the pyruvate in acetaldehyde, which is turned into ethanol (an alcohol). They throw away the alcohol because it is poisonous.
3.5 - Photosynthesis
Click Here for a general video on Photosynthesis
Click Here for a more specific video on Photosynthesis
Photosynthesis has Two Steps:
Light Dependent Reactions
Photolysis
In the thylakoid membrane
Photophosphorylation
Electron transport chain
In the thylakoid membrane
Photophosphorylation
Light Independent Reactions
The Calvin cycle
In the Stroma
Chlorophyll is the pigment in chloroplast that makes plants green. They do not absorb green light as if they absorbed all colors they would overheat and denature,
Photolysis in the thylakoid membrane is where water is split into 2 H+ ions, an electron, and an O atom (½ O2) using sunlight. Photosystems are the proteins within the thylakoid membrane which store chlorophyll. They are shaped like a flower. Electrons enter the photosystem from hydrolysis which releases heat and fluorescence. It “hops” to the top of the photosystem and is passed through the electron transport chain. The electron transport chain pumps the H+ into the stroma, which takes energy from the electron to do this. The sun shines into the electrons when they enter photosystem I, re-energizing them. They can then turn NAD+ into NADH. There is ATP synthase which makes ATP for the Calvin cycle using the H+ ions pumped into the stroma by the electron transport chain.
H2O + Light + NAD+ → O2 + ATP (For Calvin cycle) + NADPH (For Calvin cycle)
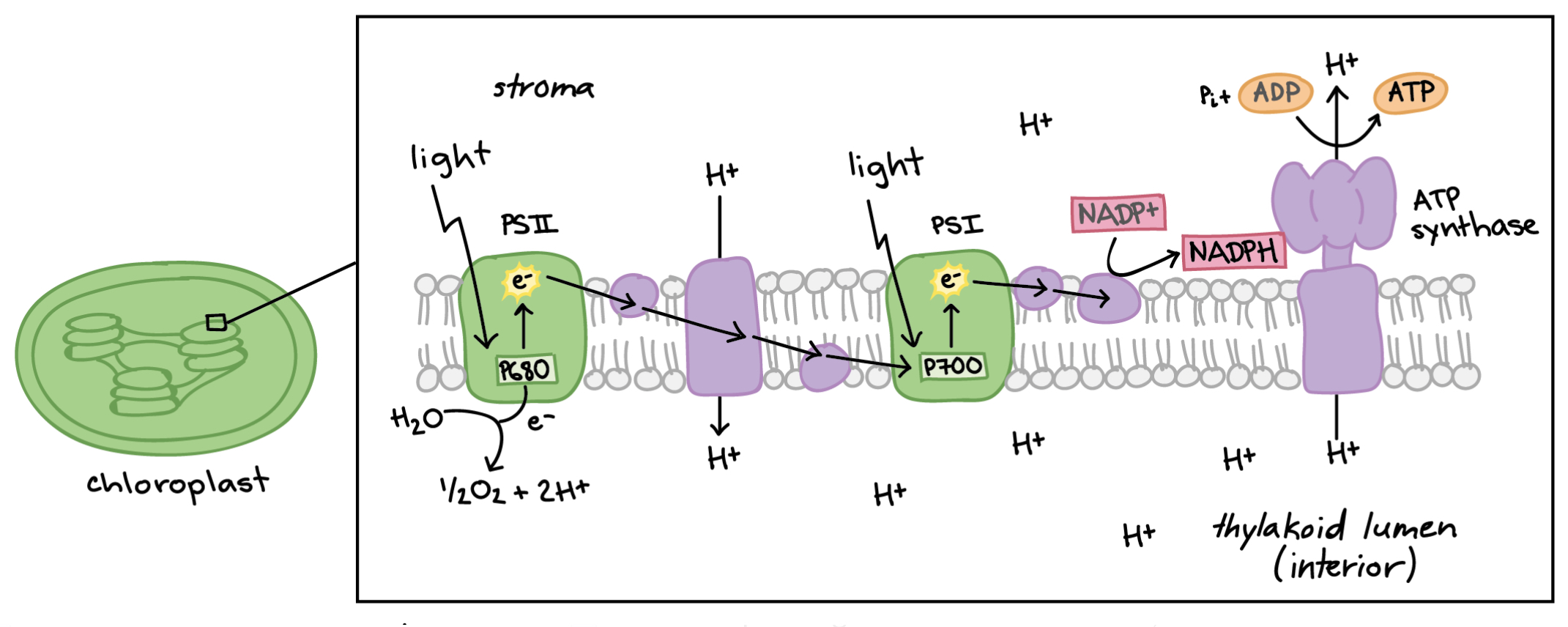
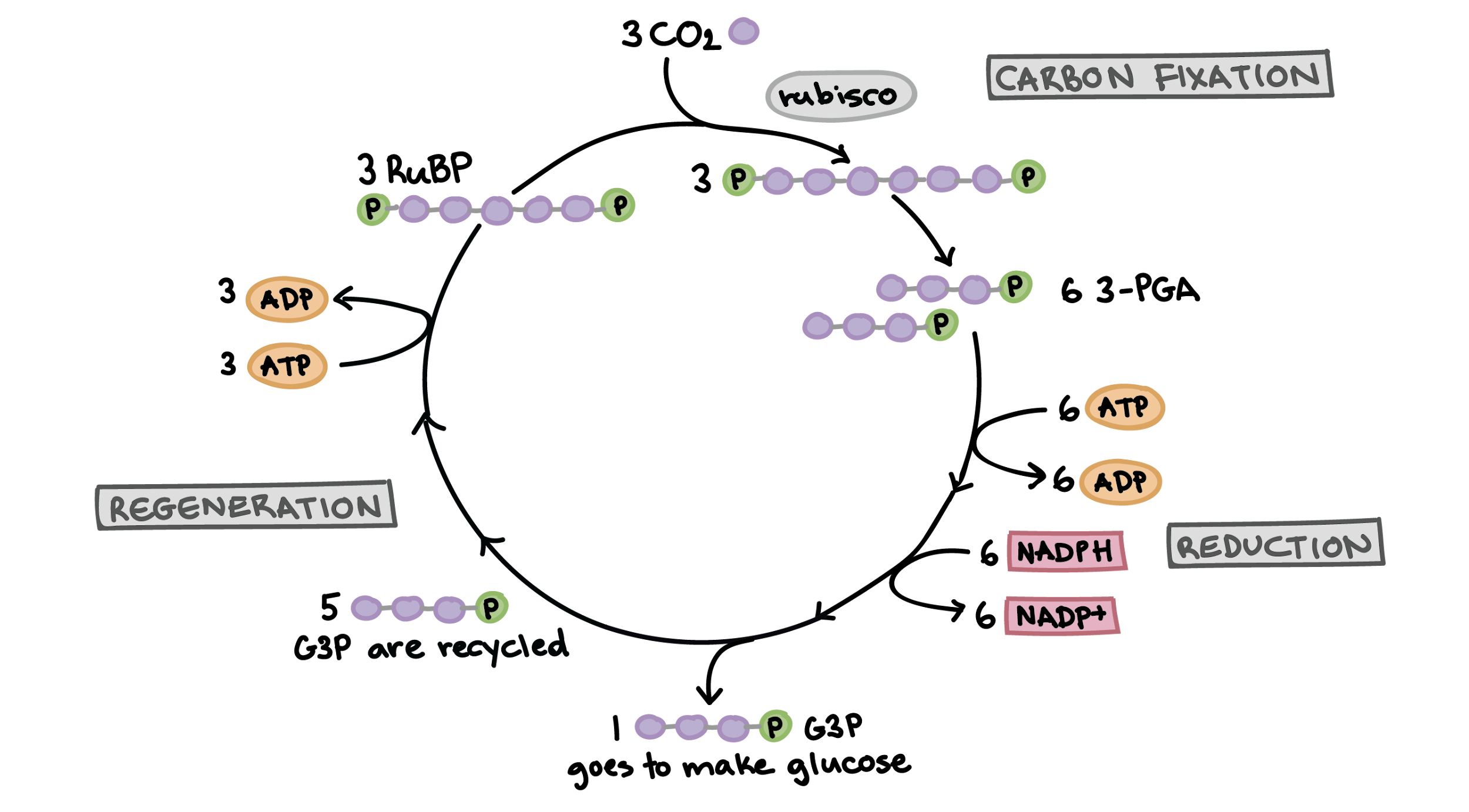
3 Molecules of CO2 is taken in the Calvin cycle. This combines with RUBP sugar with the help of RuBisCO. ATP and NADPH (turned into NADP) is consumed to make 6 groups of 3 carbon. 1 is removed to make glucose, and the other 6 are recycled using more ATP to make RUBP sugar so the cycle can repeat.
Once the cycle has gone through twice, the 2 product G3P carbons are combined in the cytosol, making glucose.
3.7 - Plant Fitness
Click Here for a video on plant fitness (start at 8:35)
Photorespiration is bad for plants. When plants don’t get enough water, they are forced to close their stomata to prevent water loss, but this prevents oxygen from entering and carbon dioxide from leaving. The enzyme RuBisCO will mistakenly pick up O2 instead of CO2 for the Calvin cycle and combine it with RuBP sugar. This ends up destroying the cycle and wasting the materials. Some plants have evolved to combat this.
C3 Plants
C3 plants YOLO it. They’ve got no plan for preventing photorespiration, they just hope and pray. (And die) Because of this, they don’t often live in deserts or other risky environments. Ex: Rice, wheat, soybeans
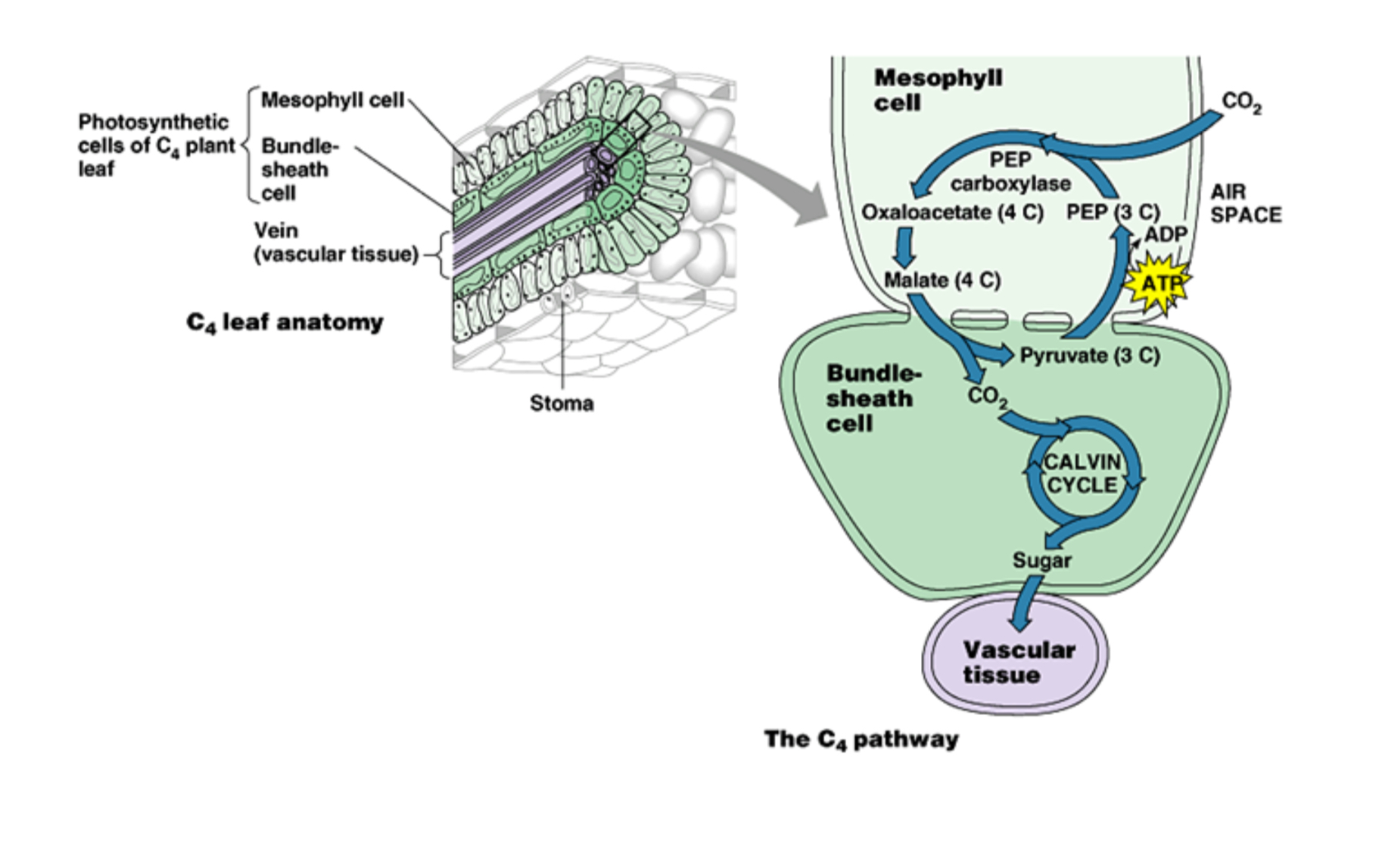
C4 Plants
C4 plants are the only plants that have a bundle sheath. The bundle sheath is a collection of cells where RuBisCO resides, which is further away from where O2 is, making it less likely for the two to come in contact and mess up the Calvin cycle.
CAM Plants
CAM plants are the most common in the desert. These plants only open their stomata at night, when water loss is less likely. They bind CO2 to other molecules to keep it safe. During the day they close their stomata and free the CO2, so they have enough CO2 to last the whole day without photorespiration.