1 Foundations of Biology
work in progress!
1.1 Studying biology: the practice of science
- ^^Biology^^ - the study of living organisms
- Scientific method is used to design and perform experimental investigations.
- Well-designed investigations take into consideration current observations and previous results.
The importance of observation
- Observations can reveal how organisms function and how they interact with others + the environment.
- Observations take advantage of human senses and apparatus for more accurate results.
- They can be interpreted differently based on what one already knows/has experienced.
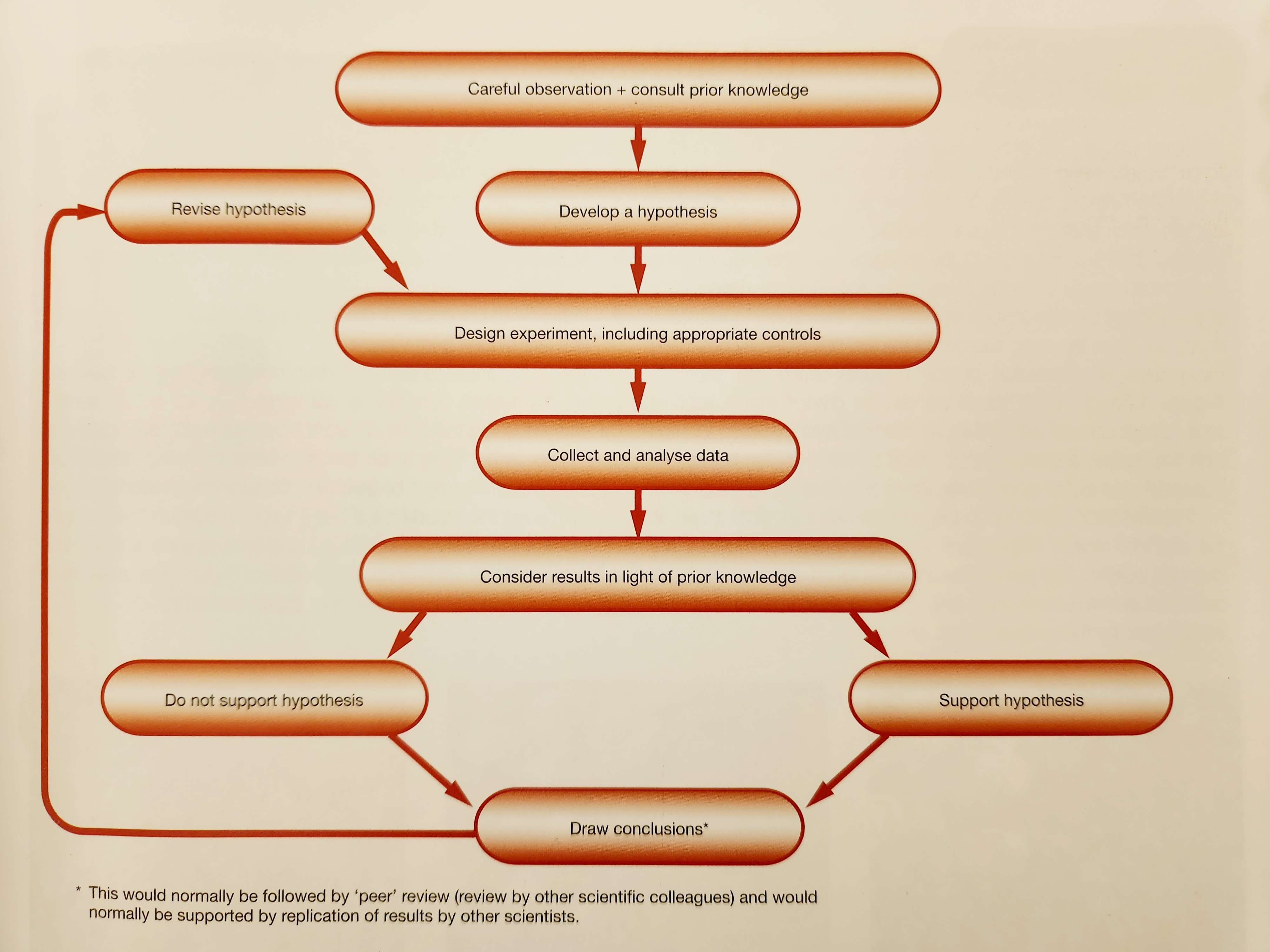
Learning by experimentation: the scientific method
- Scientists observe what is already known, then ask questions (“why?”); an experimental approach to the study of science.
- ^^Hypothesis^^- a potential justification/explanation for things that are observed.
- Can be used to predict events/behaviour.
- Tested in experiments to determine accuracy; the hypothesis is rejected if inaccurate, supported if accurate.
- ^^Theory^^ - if the hypothesis has been proven as correct under all the conditions that it has been tested in, it becomes this (AKA principle)
Asking the right questions: making hypotheses
- Hypotheses have to be testable, but even if it can’t be tested, it doesn’t mean it’s not correct.
- Gather information that is relevant to proving (or disproving) the hypothesis.
Choosing the right method
- Methods have to be reliable; described in sufficient detail to allow for repetitions of the experiment.
- If similar results cannot be obtained upon repetition, it is considered unreliable.
- Avoid personal bias; be objective when collecting and analysing data.
- Results should be clearly stated, separate from discussion of results.
- Multiple trials should be conducted, to prove that results were not because of a one-time fluke.
- Experiments and their results need to be able to replicated in order to be validated.
The need for experimental controls
- Variables that can affect experiment outcomes:
- Time of day
- Temperature
- Amount of light
- Season
- Level of noise
- ^^Independent variable^^ - the variable that is being tested. (AKA experimental)
- ^^Dependent variable^^ - the variable that is being measured when the independent variable changes.
- ^^Controlled variable^^ - the variables that are kept constant between experiments
- ^^Control group^^ - a secondary experiment that is identical to the first, bar the single experimental variable being tested.
- As a controlled experiment, it means that one variable at a time can be tested and its effects can be analysed.
- Used to eliminate the effects that random factors have on results.
Making valid conclusions
- Valid conclusions depend on reliability of results and their interpretations.
- ^^Speculations^^ - suggestions on what may be occurring based on results.
^^Conclusion^^ - statement based on the observations and measurements.
Limitations of the scientific method
- Can only be applied to hypotheses that are testable, and to questions that can be answered.
- e.g it is impossible to conduct experiments around ‘life after death’
- Cannot be used to test moral or ethical issues, but can predict environmental/biological impacts.
1.2 Important principles in biology
^^Biological principles^^ - theories that are supported by immense amounts of evidence, that make it unlikely for it to be disproved in the future.
- Relevant to the way that almost all living organisms function.
“Organisms are living things”
Organisms are made of cells
- ^^Cell theory^^ - a theory which states that all organisms are made of cells, that all cells come from pre-existing cells, and that the cell is the smallest living organisational unit.
- All cells have a cell membrane that encloses the interior fluid, cytoplasm.
- All cells have DNA as genetic material.
Evolution explains diversity
- Similarities, differences and geographic distribution of organisms → organisms have changed over time.
- ^^Phylogeny^^ - study of evolutionary relationships between organisms.
- ^^Scientific classification^^ - hierarchy of names based on phylogenetic relationships that encompasses all organisms.
Characteristics of organisms
- Common to all organisms, no matter whether plant, animal, fungi, protist or bacteria.
- Made of cells
- Chemically complex and highly organised
- Exchange energy and matter in their environment
- Sense and respond to stimuli
- Grow and reproduce
- Evolve
Common requirements for life
- All life requires a source of energy.
- Amount of energy depends on organism type, stage of growth, activity level and reproductive state.
- All life requires nutrients and water for growth, maintenance and repair.
- Waste is produced as a result of the latter processes.
- Simplicity of waste excretion depends on size of organism
- All life is composed of water, organic compounds (proteins, carbs, lipids, vitamins) and minerals.
- All life requires the ability to sense and respond to stimuli in their internal and external environments.
Organisms are adapted to their environment
- Over time, species become adapted to their external environment
- ^^Natural selection^^ - individuals with features most suited to their environment survive and pass those features down to their offspring.
- Inherited behaviour and functions make organisms suited for survival in their environment.
1.3 The composition of organisms
- 92 types of naturally occurring elements on Earth (NB. this is approximate and may have changed from the years this textbook was published)
- ^^Organic compounds^^ - complex compounds composed of carbon and hydrogen that are produced or found in living organisms.
- ^^Inorganic compounds^^ - all other compounds that are not formed of carbon and hydrogen.
- They are still important for living organisms (e.g water, oxygen)
Inorganic compounds
Water
- Most organisms are 70-90% water.
- Chemical reactions that occur in cells happen in a watery medium.
- Water’s properties (such as pH and heat capacity) are very important in biological processes.
- Water molecules are cohesive; strong tendency to stick together.
- ^^Surface tension^^ - Bonds between surface of molecules.
- Water’s surface tension allows small insects to walk across its surface without ‘breaking’ the molecule.
- ^^Heat capacity^^ - The amount of heat needed to change the temperature of an amount of matter by 1°C.
- Water’s heat capacity is very high.
- As chemical reactions occur in the body produce heat, water present in the body can absorb said heat without heating the cells up significantly.
Oxygen and carbon dioxide
- ^^Cellular respiration^^ - the process of releasing energy from food molecules using oxygen.
- A constant supply of oxygen is needed to keep cells active.
- Although oxygen can be easily obtained from the atmosphere, solely marine animals are usually small given that oxygen is not soluble in water.
- Organisms that obtain oxygen from water are small, flat, inactive or have efficient ventilation systems (e.g gills)
- ^^Photosynthesis^^ - the process of making the organic compound glucose with a by-product of oxygen, using carbon dioxide, sunlight and water by plants.
- Carbon dioxide is converted into energy by plants in photosynthesis, and returned to the environment through organic material decay and as a result of cellular respiration.
- The “carbon cycle” between organisms and the atmosphere is essential to survival.
Nitrogen
- ^^Nitrogen fixation^^- the process performed by bacteria of converting atmospheric nitrogen into compounds that plants can be used.
- Nitrogen is a key component of all proteins and thus is needed in a relatively large amount.
Minerals
- Biologically important minerals:
- Phosphorous
- Potassium
- Calcium
- Magnesium
- Iron
- Sodium
- Iodine
- Sulphur
- Mineral ions (mineral salts) are retrieved from weathered rocks and absorbed into plant roots.
- Also found in cell cytosol, structural components such as bone, and enzyme + mineral molecules.
- Humans require more than 20 different minerals.
Organic molecules
- Four main types:
- Carbohydrates
- Lipids
- Proteins
- Nucleic acid
- Can be converted from one form to others, in places like the liver.
- Carbohydrates are converted to fats for storage when food is plentiful.
- The reverse occurs when food is no longer plentiful.
- Can be linked together into larger molecule chains.
Carbohydrates
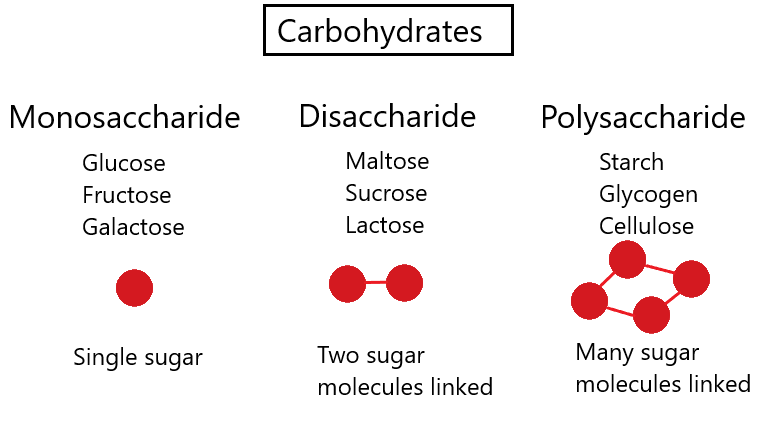
^^Carbohydrates^^ - compounds made out of carbon, hydrogen and oxygen.
Most abundant organic compound.
Important source of energy for organisms.
Plants - the carbohydrate starch is used to store energy; the carbohydrate cellulose is used to support structure.
Animals; the carbohydrate glycogen stores energy.
^^Monosaccharides^^ - subunits of carbohydrates; simple sugars.
- Glucose is an example.
- Monosaccharides have hydrogen/oxygen in the same proportions as water, meaning two hydrogens for every oxygen.
^^Disaccharides^^ - two sugars joined together.
- A molecule of water is removed.
^^Polysaccharides^^- many sugars joined together.
Lipids
- ^^Lipids^^ - fat and oil molecules that store energy.
- e.g phospholipids (cell membrane component) and steroids (hormones)
- Composed of carbon, hydrogen and vitamins in different proportions to carbohydrates.
- Smaller proportions of oxygen and can contain other elements (e.g nitrogen).
Proteins
- Thousands of differing types of proteins; functions vary widely.
- Each kind of organisms have own unique proteins.
- e.g some are hormones, some are carrier molecules.
- All are composed of carbon, hydrogen, oxygen and nitrogen.
- May also contain sulphur, phosphorus and other elements.
- Composed in chains of units called amino acids.
- ^^Peptide bonds^^ - the chemical links between amino acids in proteins.
- ^^Proteomics^^- the study of all proteins in an organism.
Nucleic acids
- ^^Nucleic acids^^- genetic material of all organisms.
- Two types: DNA (deoxyribonucleic acid) and RNA (ribonucleic acids)
- DNA contains instructions to assemble new proteins from amino acids.
- RNA plays a role in protein manufacture.
- Composed of subunits called nucleotides.
Vitamins
- ^^Vitamins^^ - Organic materials used by animals in small amounts.
- Used merely for normal functions.
- Can be naturally synthesised, but other vitamins must be obtained in diet (e.g humans must obtain Vitamin C in our diet because we cannot ‘create’ them ourselves)
- Vitamins can be used to make enzymes.