3. X-Ray Imaging
How it Works
X-ray particles are called photons
X-ray photons are delivered in packets called quanta.
If the particle energy is greater than the binding energy of the electron, then the photons
are capable of ionizing atoms.Diagnostic radiation is typically in the range of 100 nm to about 0.01 nm, or from 12 eV to 125
keV.
Components
The number of X-ray photons produced depends on the number of electrons striking the target material (so tube current)
The anode is made of either tungsten or molybdenum. The cathode is composed of two parts: the filament made of tungsten, and a focusing cup.
A change in filament current changes the intensity of the X-ray photons.
The X-ray beam coming off the cathode material is polychromatic.
Filtering out the undesired portion of the X-ray spectrum can substantially reduce the radiation dose delivered to the patient.
Math
c = λ * f
c = 3E8 m/s
1 angstrom = 10E-10 m
High frequency range is from 3E16 to 3E19 Hz
E = h * f
h = Plank’s constant = 6.63E-34 J*s = 4.13E-18 keV*s
f is frequency, or ν (Greek letter nu)
eV is an electron volt, a unit of energy representing the amount of energy one electron can obtain from accelerating between the potential difference of 1 volt
1 V = 1.602E-19 columbs = 1.602E-19 J
A pjoton with 3E18 Hz frequency has what energy?
4.13E-18 keV*s x 3E-18 Hz = 12.39 keV
Ionization in X-Rays
Simplest atom to ionize is an H atom (only 1 e-, super easy to ionize because we have a lot of H in our bodies)
If it can ionize, it has enough energy to eject an electron
13.6 eV is enough to kick out an electron, and is the threshold of ionizing
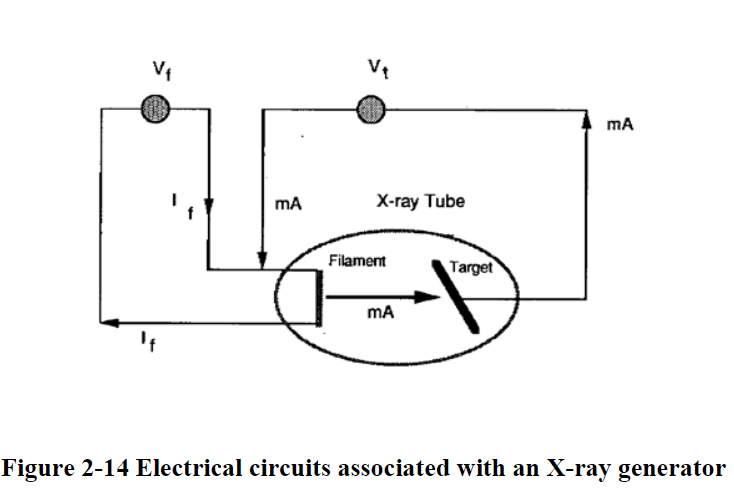
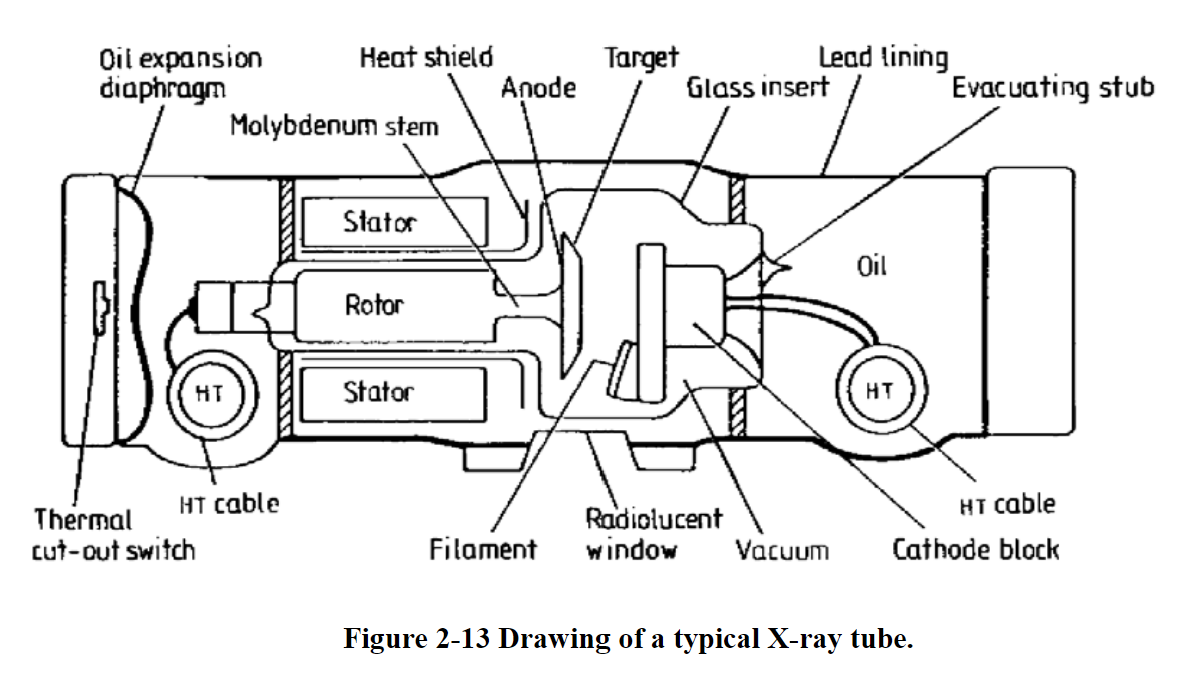
X-Ray Generation
X-rays are generated from an x-ray tube
High potential difference between cathode and anode
Acelerated electrons from a heated filament
Electrons strike the target (sometimes tungsten
Heat and x-rays are generated
99% of generated energy goes to heat
Electrons interact with the target material mainly in 2 ways to generate radiation…. Braking and Characteristic
Braking “Bremsstrahlung” Radiation
Electrons are slowed down (lose E)
Change on energy is emitted as photon energy
Generally, more photons in lower energy
Max energy is related to max kV across tube
E tube = kV * e
Characteristic Radiation
Electron strikes another inner shell electron
Inner electron is ejected with lower energy
Electrons reconfigure to fill the void
Photon is produced with specific photon energy
Photon energy depends on the shell (closer to nucleus = more E)
X-Ray Spectrum
Spectrum can also be characterized by its “effective energy” defined as the energy of a mono-energetic beam with the same penetrating ability
Effective energy is a weighted sum of the spectrum
Filtration whether intended or not, increases the effective energy of spectrum
Number of photons is the “quantity” of the x-ray beam
Energy level of the beam is the “quality”
How Might X-Rays Interact with Matter?
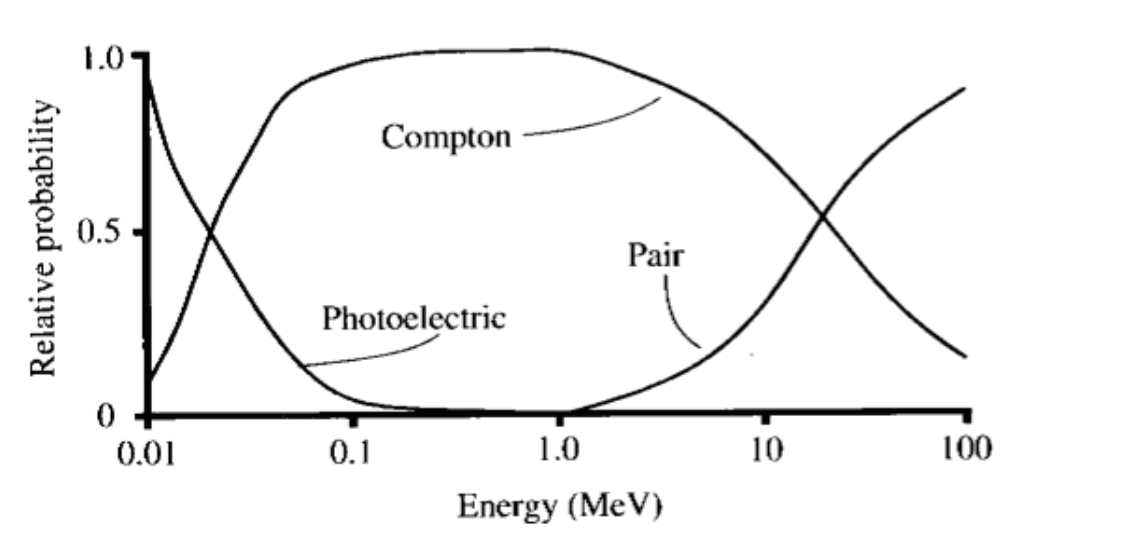
Coherent (Rayleigh) scattering
Photon bounces off in a new direction with little energy change
The electric field of the incident photon’s EM wave expends energy by making all of the electrons in the atom to oscillate in phase
Atom’s electron cloud then radiates the energy as a scattered photon
Coherent scattering is used mostly with low energy diagnostic x-rays (mammography, thyroid scans)
Electrons are not ejected so ionization does not occur
Compton scattering
If it’s above 30keV with soft tissue, it’s probably compton scattering
Steps
Photon interacts with an electron (usually valence) and only some energy from the photon goes to the electron
Photon moves on with reduced energy and new direction
Electron is ejected
Energy of the initial photon must be equal to the energy of the scattered photon + energy of ejected electron
More dense the tissue = more likely Compton scattering occurs
Compton scattering makes up most of the background noise & tissue damage
If the initial energy is low, then the scattered energy doesn’t matter on the scattering angle
If the initial energy is high, the scattered energy is higher for a smaller scattering angle
Scattered photons with higher energies will continue in pretty much the same direction
Compton scattering in which a photon is not absorbed but rather scattered. The photon energy is reduced, and an electron is ejected. This is the major source of noise in X-ray (and CT) images.
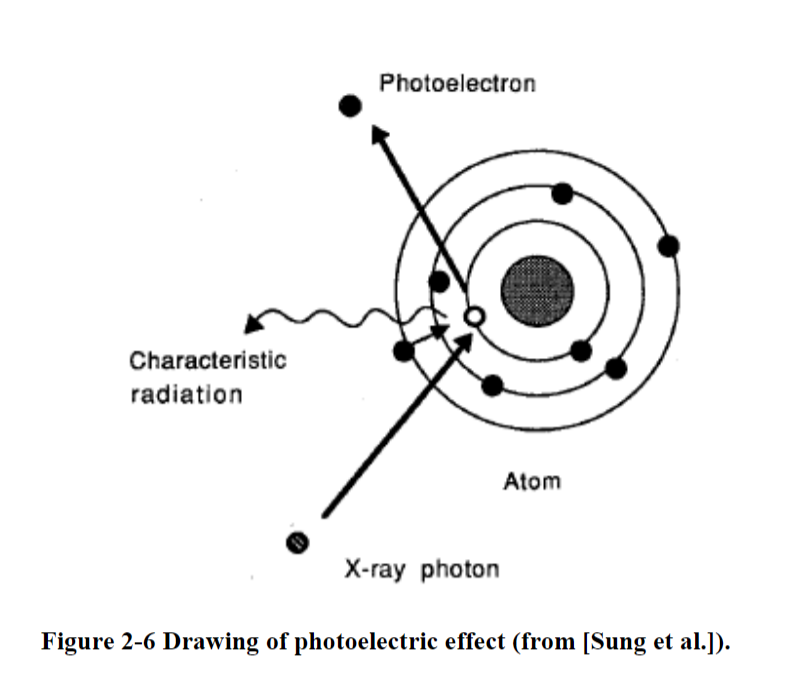
Photoelectric effect
In the photoelectric effect, all of the initial energy is transferred to an electron
Photoelectric effect in which a photon is absorbed, characteristic radiation is emitted along with photoelectrons, and possibly Auger electrons.
Steps
All photon energy transfers to electron
Electron ejects
Electron becomes a photoelectron
Energy of the photoelectron is the energy of the initial photon minus the energy it took to bind to the orbital electron
Called an Auger electron
A lower orbital electron will jump up to take its place
Energy needs to decrease now, so energy is given off as fluorescent energy
Probability of characteristic x-ray emission (dangerous) decreases as the atomic number of the absorber atom decreases (less protons = less possibility of radiation)
Soft tissue has lower atomic number so it’s not super frequent
Probability of characteristic x-ray emission also decreases with increasing photon energy
Pair Production
Pair production can occur when the energy of the incident photon exceeds 1.02 MeV
Steps
High energy photons are absorbed by a nucleus
A positron (positive electron, a form of anti-matter) is emitted with an electron
Energy above 1.02 MeV goes to the electron as kinetic energy
The positron and electron interact and shoots oppositely directed 511 keV annihilation photons
Unusual because it takes so much energy
Describes the same anti-matter formation used in PET scans
Pair production in which a photon is absorbed by the nucleus, a positron is emitted, and an electron is ejected.
Photo-disintegration
Interaction of an incident photon with a nucleus, which produces one (or more) ejected nuclear particle
One element becomes a different element
Super unusual so it takes so much energy
 Knowt
Knowt
