3. X-Ray Imaging
How it Works
- X-ray particles are called photons
- X-ray photons are delivered in packets called quanta.
- If the particle energy is greater than the binding energy of the electron, then the photons \n are capable of ionizing atoms.
- Diagnostic radiation is typically in the range of 100 nm to about 0.01 nm, or from 12 eV to 125 \n keV.
Components
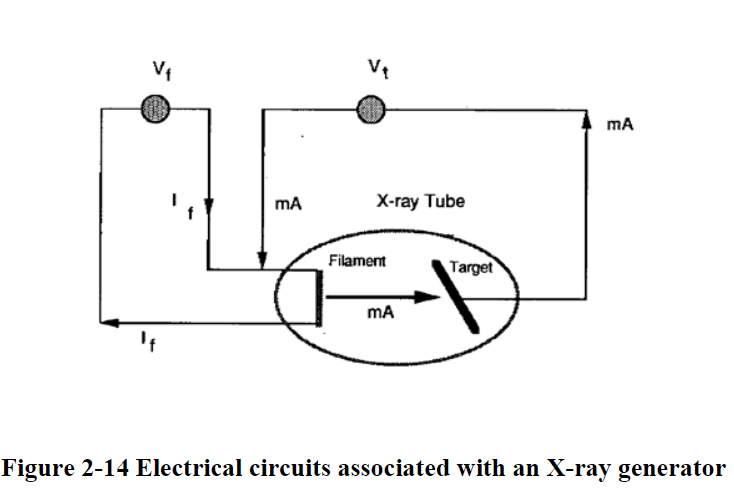
The number of X-ray photons produced depends on the number of electrons striking the target material (so tube current)
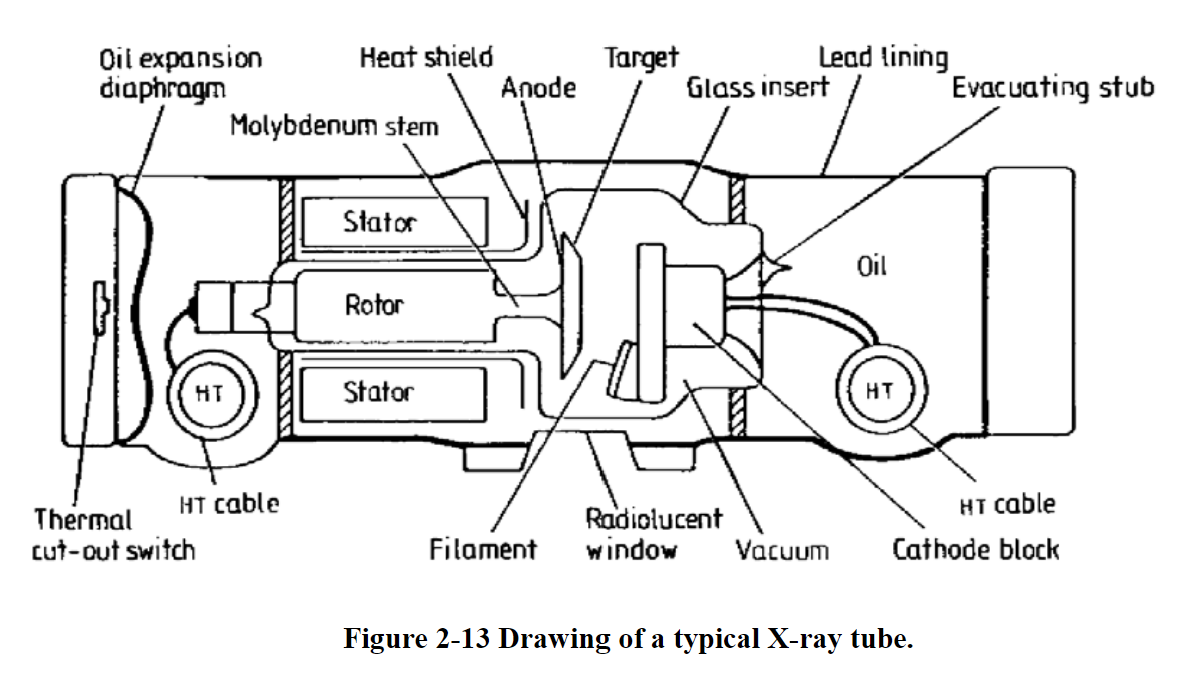
The anode is made of either tungsten or molybdenum. The cathode is composed of two parts: the filament made of tungsten, and a focusing cup.
A change in filament current changes the intensity of the X-ray photons.
The X-ray beam coming off the cathode material is polychromatic.
- Filtering out the undesired portion of the X-ray spectrum can substantially reduce the radiation dose delivered to the patient.
Math
- c = λ * f
- c = 3E8 m/s
- 1 angstrom = 10E-10 m
- High frequency range is from 3E16 to 3E19 Hz
- E = h * f
- h = Plank’s constant = 6.63E-34 J*s = 4.13E-18 keV*s
- f is frequency, or ν (Greek letter nu)
- eV is an electron volt, a unit of energy representing the amount of energy one electron can obtain from accelerating between the potential difference of 1 volt
- 1 V = 1.602E-19 columbs = 1.602E-19 J
- A pjoton with 3E18 Hz frequency has what energy?
- 4.13E-18 keV*s x 3E-18 Hz = 12.39 keV
Ionization in X-Rays
- Simplest atom to ionize is an H atom (only 1 e-, super easy to ionize because we have a lot of H in our bodies)
- If it can ionize, it has enough energy to eject an electron
- 13.6 eV is enough to kick out an electron, and is the threshold of ionizing
X-Ray Generation
- X-rays are generated from an x-ray tube
- High potential difference between cathode and anode
- Acelerated electrons from a heated filament
- Electrons strike the target (sometimes tungsten
- Heat and x-rays are generated
- 99% of generated energy goes to heat
- Electrons interact with the target material mainly in 2 ways to generate radiation…. Braking and Characteristic
Braking “Bremsstrahlung” Radiation
- Electrons are slowed down (lose E)
- Change on energy is emitted as photon energy
- Generally, more photons in lower energy
- Max energy is related to max kV across tube
- E tube = kV * e
Characteristic Radiation
- Electron strikes another inner shell electron
- Inner electron is ejected with lower energy
- Electrons reconfigure to fill the void
- Photon is produced with specific photon energy
- Photon energy depends on the shell (closer to nucleus = more E)
X-Ray Spectrum
- Spectrum can also be characterized by its “effective energy” defined as the energy of a mono-energetic beam with the same penetrating ability
- Effective energy is a weighted sum of the spectrum
- Filtration whether intended or not, increases the effective energy of spectrum
- Number of photons is the “quantity” of the x-ray beam
- Energy level of the beam is the “quality”
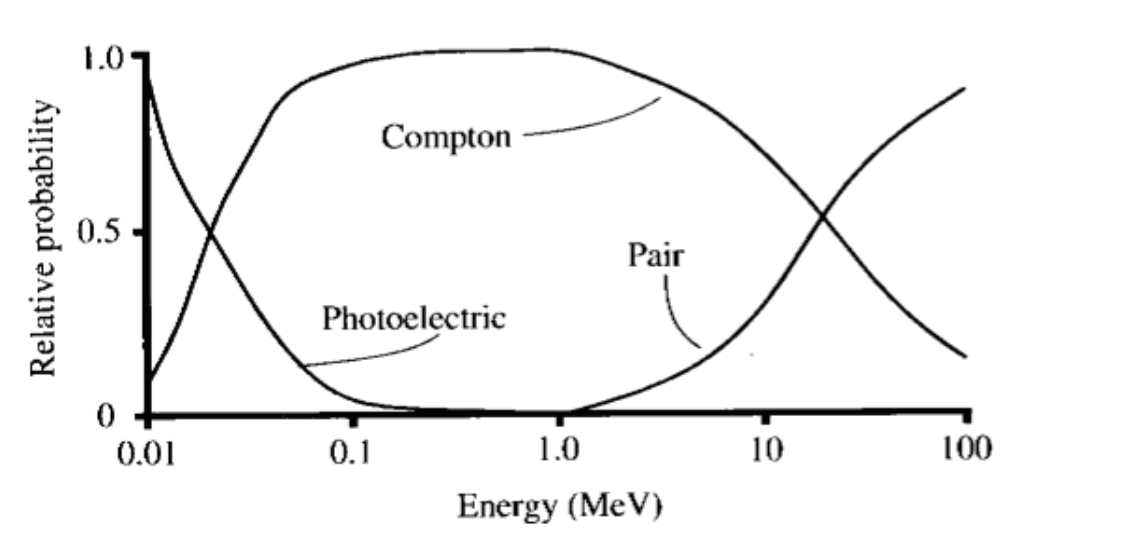
How Might X-Rays Interact with Matter?
Coherent (Rayleigh) scattering
- Photon bounces off in a new direction with little energy change
- The electric field of the incident photon’s EM wave expends energy by making all of the electrons in the atom to oscillate in phase
- Atom’s electron cloud then radiates the energy as a scattered photon
- Coherent scattering is used mostly with low energy diagnostic x-rays (mammography, thyroid scans)
- Electrons are not ejected so ionization does not occur
Compton scattering
- If it’s above 30keV with soft tissue, it’s probably compton scattering
- Steps
- Photon interacts with an electron (usually valence) and only some energy from the photon goes to the electron
- Photon moves on with reduced energy and new direction
- Electron is ejected
- Energy of the initial photon must be equal to the energy of the scattered photon + energy of ejected electron
- More dense the tissue = more likely Compton scattering occurs
- Compton scattering makes up most of the background noise & tissue damage
- If the initial energy is low, then the scattered energy doesn’t matter on the scattering angle
- If the initial energy is high, the scattered energy is higher for a smaller scattering angle
- Scattered photons with higher energies will continue in pretty much the same direction
- Compton scattering in which a photon is not absorbed but rather scattered. The photon energy is reduced, and an electron is ejected. This is the major source of noise in X-ray (and CT) images.
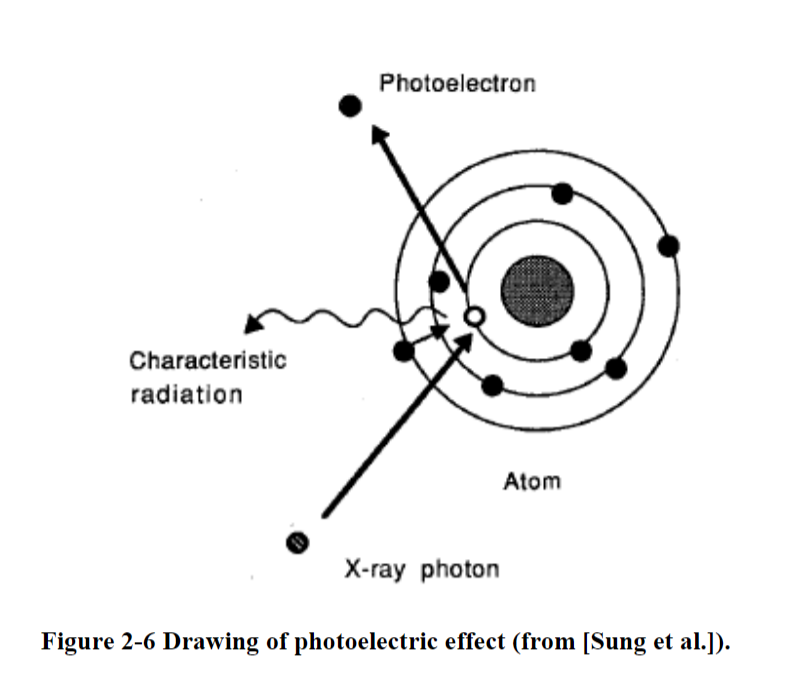
Photoelectric effect
In the photoelectric effect, all of the initial energy is transferred to an electron
Photoelectric effect in which a photon is absorbed, characteristic radiation is emitted along with photoelectrons, and possibly Auger electrons.
Steps
- All photon energy transfers to electron
- Electron ejects
- Electron becomes a photoelectron
- Energy of the photoelectron is the energy of the initial photon minus the energy it took to bind to the orbital electron
- Called an Auger electron
- A lower orbital electron will jump up to take its place
- Energy needs to decrease now, so energy is given off as fluorescent energy
Probability of characteristic x-ray emission (dangerous) decreases as the atomic number of the absorber atom decreases (less protons = less possibility of radiation)
Soft tissue has lower atomic number so it’s not super frequent
Probability of characteristic x-ray emission also decreases with increasing photon energy
Pair Production
- Pair production can occur when the energy of the incident photon exceeds 1.02 MeV
- Steps
- High energy photons are absorbed by a nucleus
- A positron (positive electron, a form of anti-matter) is emitted with an electron
- Energy above 1.02 MeV goes to the electron as kinetic energy
- The positron and electron interact and shoots oppositely directed 511 keV annihilation photons
- Unusual because it takes so much energy
- Describes the same anti-matter formation used in PET scans
- Pair production in which a photon is absorbed by the nucleus, a positron is emitted, and an electron is ejected.
Photo-disintegration
Interaction of an incident photon with a nucleus, which produces one (or more) ejected nuclear particle
One element becomes a different element
Super unusual so it takes so much energy