🤔AP BIO Chapter 16 - Development, Stem Cells, and Cancer
Overview
16.1 A program of differential gene expression leads to the different cell types in a multicellular organism
16.2 Cloning of organisms showed that differentiated cells could be “reprogrammed” and ultimately led to the production of stem cells
16.3 Abnormal regulation of genes that affect the cell cycle can lead to cancer
BIG IDEAS: The timing and coordination of key events are necessary for the normal development of an organism (Big Idea 2). Different control factors and enzymes (Big Idea 4) regulate gene expression and cell differentiation (Big Idea 3), which if altered can result in abnormal development or cancer
<<Model Organism:<<
A particular species chosen for research into broad biological principles because it is representative of a larger group ad usually easy to grow in a lab
%%16.1 - A program of differential gene expression leads to the different cell types in a multicellular organism%%
Embryonic cells undergo differentiation, becoming specialized in structure and function. Morphogenesis encompasses the processes that give shape to the organism and its various structures. Cells differ in structure and function not because they contain different genomes but because they express different genes
Localized cytoplasmic determinants in the unfertilized egg are distributed differentially to daughter cells, where they regulate the expression of genes that affect those cells’ developmental fates. In the process called induction, signaling molecules from embryonic cells cause transcriptional changes in nearby target cells
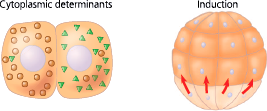
Differentiation is heralded by the appearance of tissue-specific proteins, which enable differentiated cells to carry out their roles
Apoptosis is a type of programmed cell death in which cell components are disposed of in an orderly fashion, without damage to neighboring cells. Studies of the soil worm Caenorhabditis elegans showed that apoptosis occurs at defined times during embryonic development. Related apoptotic signaling pathways exist in the cells of humans and other mammals, as well as yeasts
In animals, pattern formation, the development of a spatial organization of tissues and organs, begins in the early embryo. Positional information, the molecular cues that control pattern formation, tells a cell its location relative to the body’s axes and to other cells. In Drosophila melanogaster, gradients of morphogens encoded by maternal effect genes determine the body axes. For example, the gradient of Bicoid protein determines the anterior-posterior axis
<<Zygtote:<<
A fertilized egg
Mitosis produces ___ cells
- identical
<<Differentiation:<<
The process by which a cell or group of cells becomes specialized in structure and function
<<Morphogenesis:<<
The development of the form of an organism and its structures
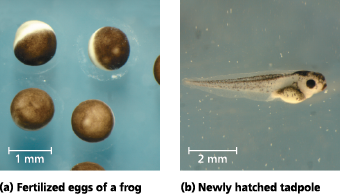
From fertilized egg to animal: What a difference four days makes - It takes just four days for cell division, differentiation, and morphogenesis to transform each of the fertilized frog eggs shown in (a) into a tadpole like the one in (b)
Almost all cells in an organism have the same genome; therefore, differential gene expression results from ___
- the genes being regulated differently in each cell type
What’s in the egg’s cytoplasm?
RNA and proteins are encoded by the mother’s DNA. They are unevenly distributed
<<Cytoplasmic Determinant:<<
A maternal substance, such as a protein or NA, that when placed into an egg influences the course of early development by regulating the expression of genes that affect the developmental fate of cells
Sources of developmental information for the early embryo
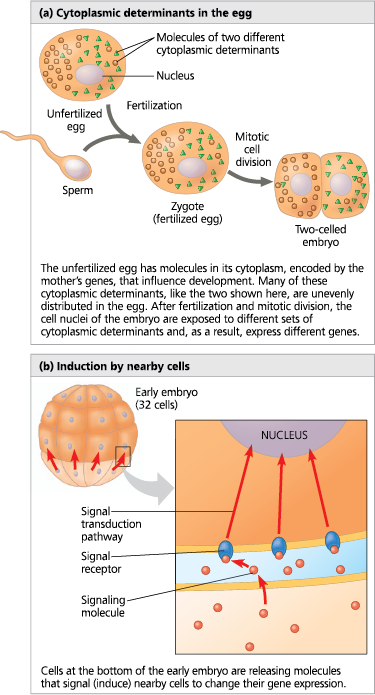
<<Induction:<<
The process in which one group of embryonic cells influences the development of another, usually by causing changes in gene expression
<<Determination:<<
The progressive restriction of developmental potential whereby the possible fate of each cell becomes more limited as an embryo develops. At the end of determination, a cell is committed to its fate
Irreversible ___ is followed by _
- determination; differentiation
<<Tissue-Specific Proteins:<<
Found only in a specific cell type and give the cell its characteristic structure and function
The first sign of differentiation is the ___ for tissue-specific proteins
- appearance of mRNAs
<<Myoblasts:<<
After determination has occurred they churn large amounts of muscle-specific proteins and fuse to form mature, elongated, multinucleate skeletal muscle cells
MyoD also stimulates expression of the myoD gene itself, an example of ___ feedback that perpetuates MyoD’s effect in maintaining the cell’s differentiated state
- positive
Determination and differentiation of muscle cells - Skeletal muscle cells arise from embryonic cells as a result of changes in gene expression. (In this depiction, the process of gene activation is greatly simplified.)
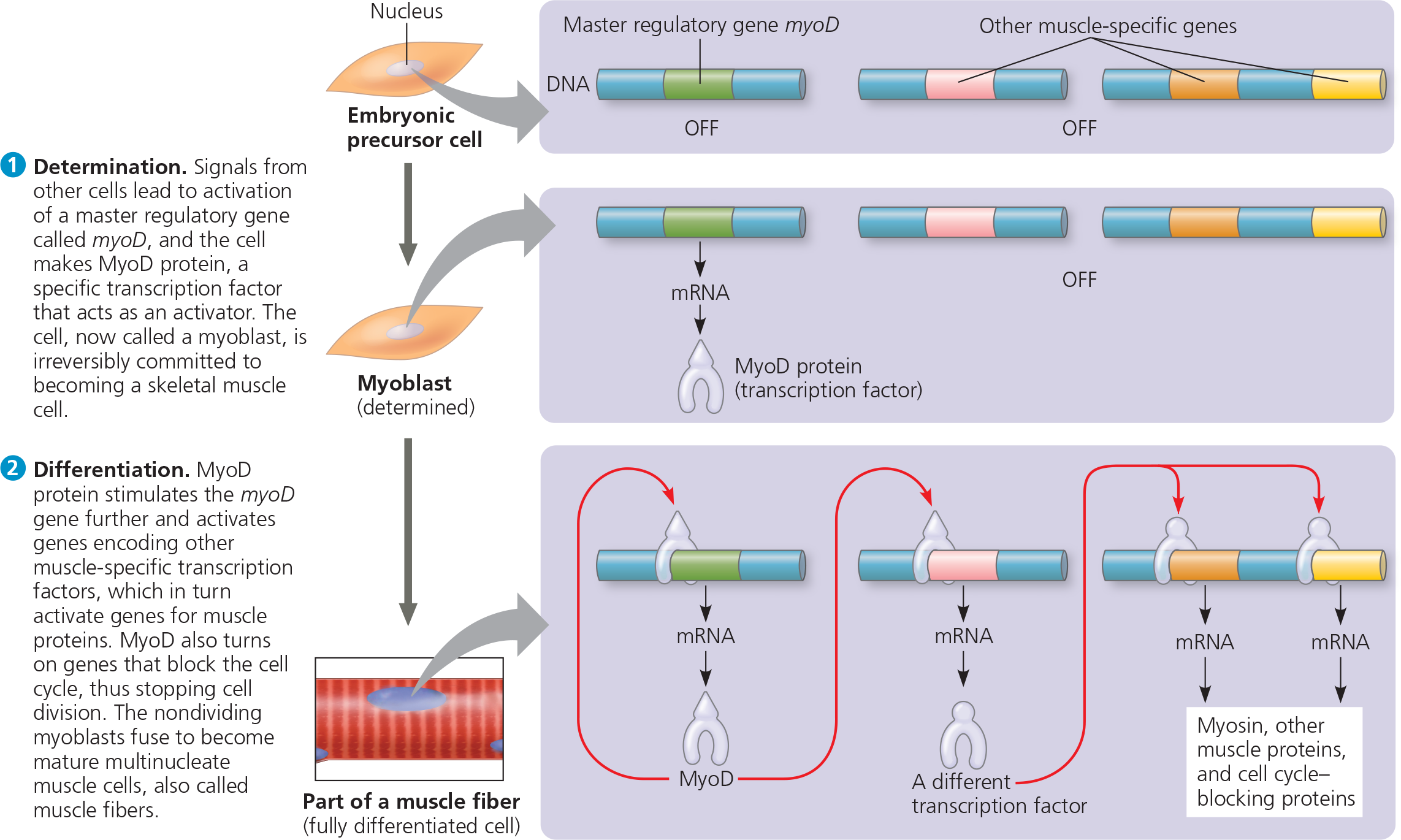
%%What would happen if a mutation in the myoD gene resulted in a MyoD protein that could not activate the myoD gene?%%
Even if the mutant MyoD protein couldn’t activate the myoD gene, it could still turn on genes for the other proteins in the pathway (other transcription factors, which would turn on the genes for muscle-specific proteins, for example). Therefore, some differentiation would occur. But unless there were other activators that could compensate for the loss of the MyoD protein’s activation of the myoD gene, the cell would not be able to maintain its differentiated state
Researchers interested in the regulation of Hoxd13 gene expression genetically engineered a set of mice that had different segments of DNA deleted upstream of the gene. They then compared levels and patterns of Hoxd13 gene expression in developing paws of 12.5-day-old mouse embryos that had the DNA deletions with gene expression seen in wild-type mouse embryos of the same age
They used two different approaches: In some mice, they extracted the mRNA from the embryonic paws and quantified the overall level of Hoxd13 mRNA in the whole paw. In another set of the same mice, they used in situ hybridization to pinpoint exactly where in the paws the Hoxd13 gene was expressed as mRNA. The particular technique that was used causes the Hoxd13 mRNA to appear blue or black, which indicates the highest mRNA levels 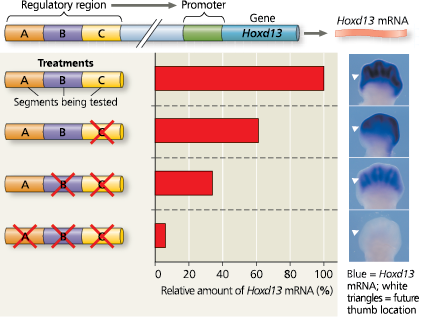
<<Apoptosis:<<
A type of programmed cell death that is brought about by the activation of enzymes that break down many chemical components in the cell
Apoptosis prevents ___ because otherwise they are _ passed on to the next generation of cells
- mutations; permanently
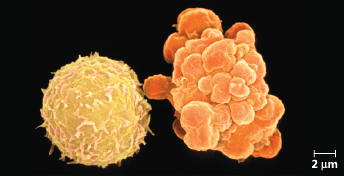
Apoptosis of a human white blood cell - We can compare a normal white blood cell (left) with a white blood cell undergoing apoptosis (right) (colorized SEM). The apoptotic cell is shrinking and forming lobes (“blebs”), which eventually are shed as membrane-enclosed cell fragments
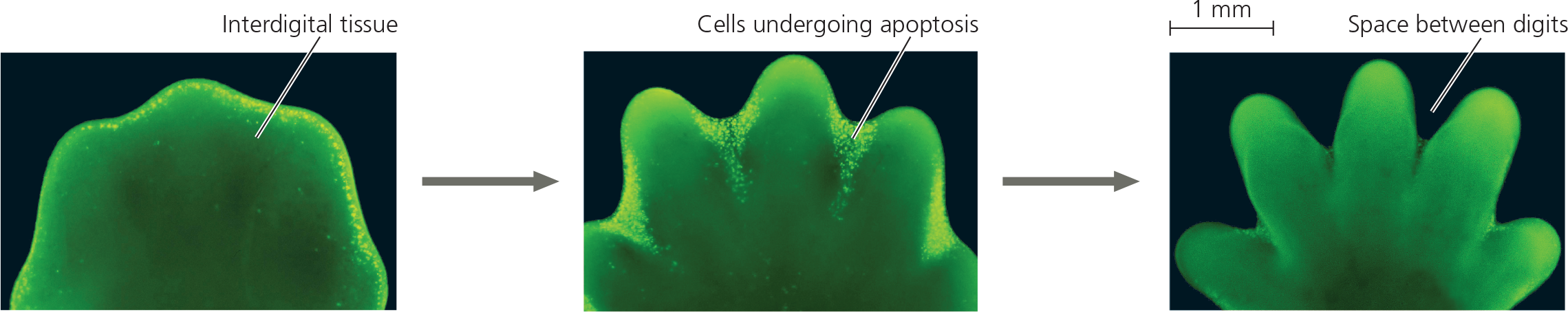
Effect of apoptosis during paw development in the mouse - In mice, humans, other mammals, and land birds, the embryonic region that develops into feet or hands initially has a solid, platelike structure. Apoptosis eliminates the cells in the interdigital regions, thus forming the digits. The embryonic mouse paws shown in these fluorescence light micrographs are stained so that cells undergoing apoptosis appear bright yellow. Apoptosis of cells begins at the margin of each interdigital region (left), peaks as the tissue in these regions is reduced (middle), and is no longer visible when the interdigital tissue has been eliminated (right). (Note that the Scientific Skills Exercise shows a different genetic process involved in mouse paw development.)
<<Body Plan:<<
The organism’s overall three-dimensional arrangement that must be established and superimposed on the differentiation process
<<Pattern Formation:<<
The development of a multicellular organism’s spatial organization, the arrangement of organs and tissues in their characteristic places in three-dimensional space
<<Positional Information:<<
Molecular cues that control pattern formation in an animal or plant embryonic structure by indicating a cell’s location relative to the organism’s body axes. These cues elicit a response by genes that regulate development
The molecular cues that control pattern formation are provided by ___
- cytoplasmic determinants and inductive signals
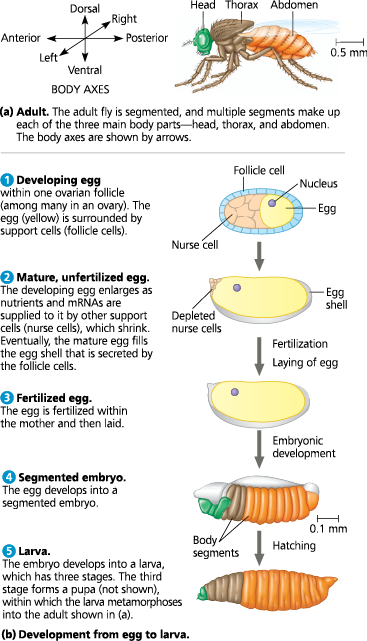
Key events in Drosophila development
<<Homeotic Gene:<<
Any of the master regulatory genes that control the placement and spatial organization of body parts in animals, plants, and fungi by controlling the developmental fate of groups of cells
Abnormal pattern formation in Drosophila - Mutations in homeotic genes cause misplacement of structures. These scanning electron micrographs contrast the head of a wild-type fly with that of a homeotic mutant (a fly with a mutation in a homeotic gene) bearing a pair of legs in place of antennae
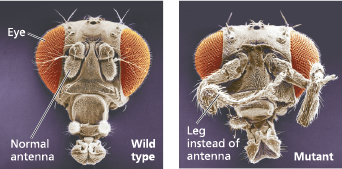
<<Embryonic Lethal:<<
A mutation with a phenotype leading to the death of an embryo or larva
What made it hard to identify all the genes that affect segment formation in Drosophila?
There’s a large number of Drosophila protein-coding genes, now known to total about 14,000. Mutations affecting segmentation would be embryonic lethals, never reproduce, and be hard to study. Cytoplasmic determinants in the egg were known to play a role in axis formation, so the researchers knew they would have to study the mother’s genes as well as those of the embryo
<<Maternal Effect Gene (Egg Polarity Genes):<<
A gene that, when mutant in the mother, results in a mutant phenotype in the offspring, regardless of the offspring’s genotype. Maternal effect genes, also called egg-polarity genes, were first identified in Drosophila melanogaster
<<Bicoid:<<
A maternal effect gene that codes for a protein responsible for specifying the anterior end in Drosophila melanogaster
<<Morphogen Gradient Hypothesis:<<
Gradients of morphogens establish an embryo’s axes and other features of its form
<<Morphogens:<<
A substance, such as the Bicoid protein in Drosophila, that provides positional information in the form of a concentration gradient along an embryonic axis
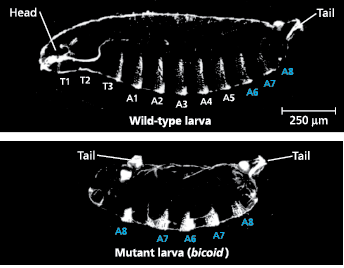
Effect of the bicoid gene on Drosophila development - A wild-type fruit fly larva has a head, three thoracic (T) segments, eight abdominal (A) segments, and a tail. A larva whose mother has two mutant alleles of the bicoid gene has two tails and lacks all anterior structures (LMs)
The researchers hypothesized that bicoid normally codes for a morphogen that specifies the head (anterior) end of the embryo. They used molecular techniques to determine whether the mRNA and protein encoded by this gene were found in the anterior end of the fertilized egg and early embryo of wild-type flies
Results: Bicoid mRNA (dark blue in the light micrographs and drawings) was confined to the anterior end of the unfertilized egg. Later in development, Bicoid protein (dark orange) was seen to be concentrated in cells at the anterior end of the embryo 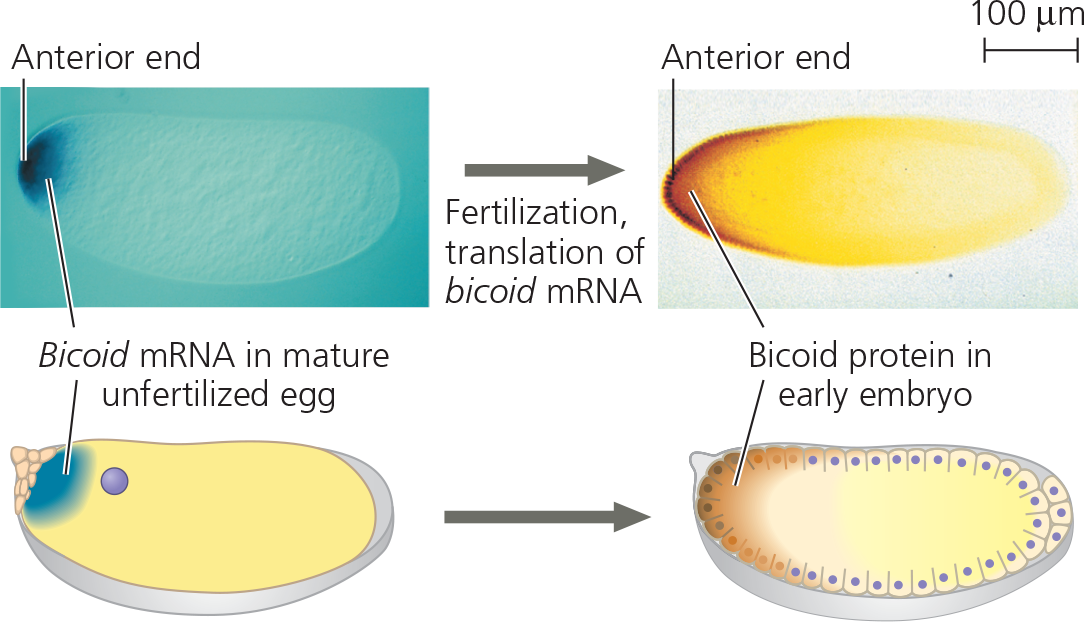
%%The researchers needed further evidence, so they injected bicoid mRNA into the anterior end of an egg from a female with a mutation disabling the bicoid gene. Given that the hypothesis was supported, what must their results have been?%%
Normal Bicoid protein would be made in the anterior end and compensate for the presence of mutant bicoid mRNA put into the egg by the mother. Development should be normal, with a head present. (This is what was observed.)
What was concluded from the experiment?
The location of bicoid mRNA and the diffuse gradient of Bicoid protein seen later are consistent with the hypothesis that Bicoid protein is a morphogen specifying formation of head-specific structures
What did the bicoid research lead to?
It led to the identification of a specific protein required for some of the earliest steps in pattern formation. It increased our understanding of the mother’s critical role in the initial phases of embryonic development
%%Mitosis gives rise to two daughter cells that are genetically identical to the parent cell. Yet you, the product of many mitotic divisions, are not composed of identical cells. Why?%%
Cells undergo differentiation during embryonic development, becoming different from each other. Therefore, the adult organism is made up of many highly specialized cell types
%%Explain how the signaling molecules released by an embryonic cell can induce changes in a neighboring cell without entering the cell%%
By binding to a receptor on the receiving cell’s surface and triggering a signal transduction pathway involving intracellular molecules such as second messengers and transcription factors that affect gene expression 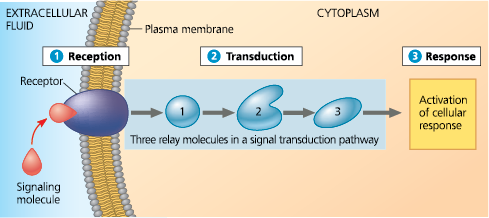
%%How do fruit fly maternal effect genes determine the polarity of the egg and the embryo?%%
The products of maternal effect genes, made and deposited into the egg by the mother, determine the head and tail ends, as well as the back and belly, of the egg and embryo (and eventually the adult fly)
%%Describe the two main processes that cause embryonic cells to head down different pathways to their final fates%%
The first process involves cytoplasmic determinants, including mRNAs and proteins, placed into specific locations in the egg by the mother. The cells that are formed from different regions in the egg during early cell divisions will have different proteins in them, which will direct different programs of gene expression. The second process involves the cell in question responding to signaling molecules secreted by neighboring cells. The signaling pathway in the responding cells also leads to a different pattern of gene expression. The coordination of these two processes results in each cell following a unique pathway in the developing embryo
%%16.2 - Cloning of organisms showed that differentiated cells could be “reprogrammed” and ultimately led to the production of stem cells%%
Studies showing genomic equivalence (that an organism’s cells all have the same genome) are the first cases of organismal cloning
Single differentiated cells from plants are often totipotent: capable of generating all the tissues of a completely new plant
Transplantation of the nucleus from a differentiated animal cell into an enucleated egg can sometimes give rise to a new animal
Certain embryonic stem cells (ES cells) from animal embryos or adult stem cells from adult tissues can reproduce and differentiate in a test tube as well as in the organism, offering the potential for medical use. ES cells are pluripotent but difficult to acquire. Induced pluripotent stem (iPS) cells resemble ES cells in their capacity to differentiate; they can be generated by reprogramming differentiated cells. iPS cells hold promise for medical research
<<Organismal Cloning:<<
A genetically identical organism from a parent
<<Stem Cell:<<
Any relatively unspecialized cell that can produce, during a single division, one identical daughter cell and one more specialized daughter cell that can undergo further differentiation
When cells “dedifferentiate“, they can give rise to ___
- all the specialized cell types of the organism
<<Totipotent:<<
Describing a cell that can give rise to all parts of the embryo and adult, as well as extraembryonic membranes in species that have them
<<Nuclear Transplantation:<<
Creating an enucleated egg and replacing it with the nucleus of a differentiated cell
Differentiated animal cell experiment results - When the transplanted nuclei came from an early embryo, the cells of which are relatively undifferentiated, most of the recipient eggs developed into tadpoles. But when the nuclei came from the fully differentiated intestinal cells of a tadpole, fewer than 2% of the eggs developed into normal tadpoles, and most of the embryos stopped developing at a much earlier stage 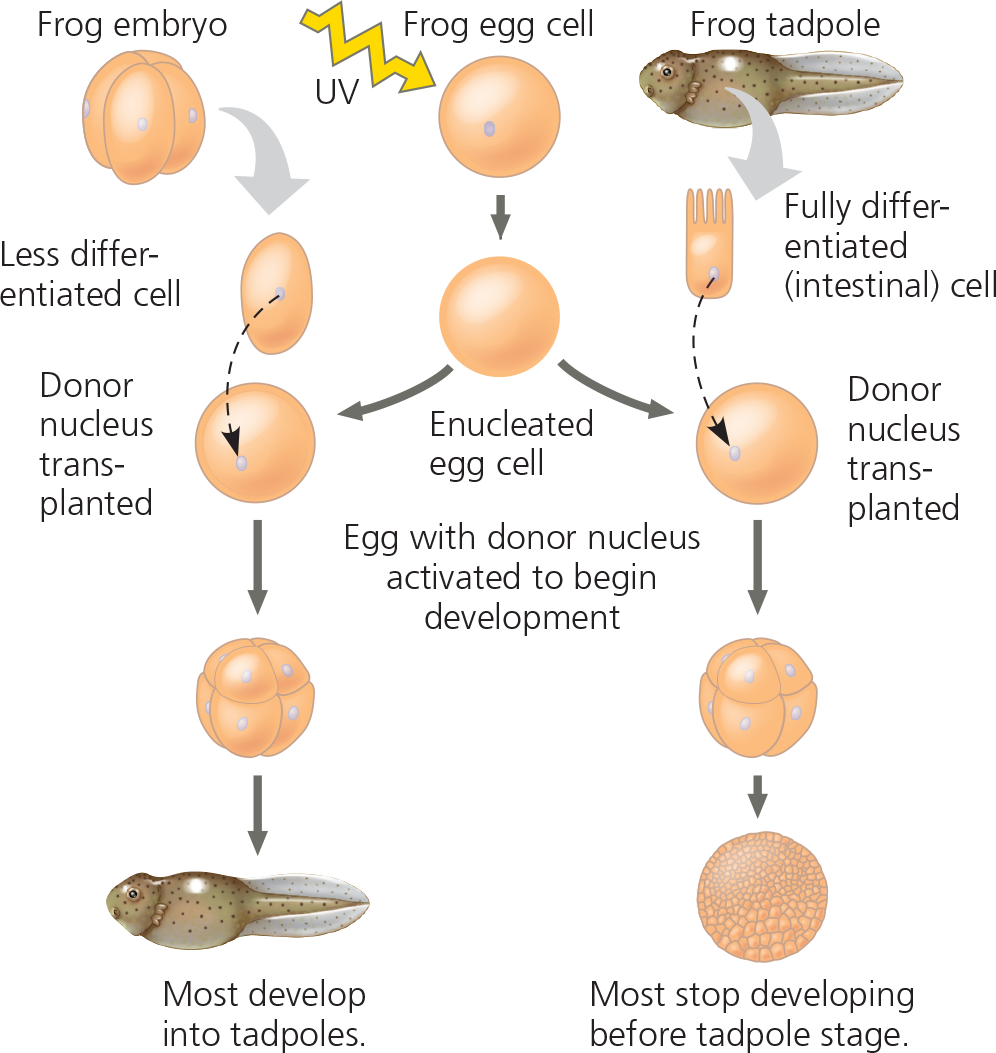
Conclusion - The nucleus from a differentiated frog cell can direct development of a tadpole. However, its ability to do so decreases as the donor cell becomes more differentiated, presumably because of changes in the nucleus
%%If each cell in a four-cell embryo were already so specialized that it was not totipotent, what results would you predict for the experiment on the left side of the figure?%%
None of the eggs with the transplanted nuclei from the four-cell embryo at the upper left would have developed into a tadpole. Also, the resulting samples might include only some of the tissues of a tadpole. The tissues that develop might differ from treatment to treatment, depending on which of the four nuclei was transplanted. (This assumes that there was some way to tell the four cells apart, as one can in some frog species.)
Something in the nucleus ___ as animal cells differentiate
- does change
What resulted from Dolly’s cloning?
Her unhealthy cells possibly reflected incomplete reprogramming of the original transplanted nucleus
<<Reproductive Cloning:<<
Cloning mammals with the goal to produce a new individual
CC (“Carbon Copy”), the first cloned cat (right), and her single parent - Rainbow (left) donated the nucleus in a cloning procedure that resulted in CC. However, the two cats are not identical: Rainbow has orange patches on her fur, but CC does not

Describe reasons for the low efficiency of cloning and the high incidence of abnormalities
In the nuclei of fully differentiated cells, a small subset of genes is turned on and expression of the rest of the genes is repressed (regulation by acetylation or methylation). This finding suggests that the reprogramming of donor nuclei requires more accurate and complete chromatin restructuring than occurs during cloning procedures. Because DNA methylation helps regulate gene expression, misplaced or extra methyl groups in the DNA of donor nuclei may interfere with the pattern of gene expression necessary for normal embryonic development
How stem cells maintain their own population and generate differentiated cells
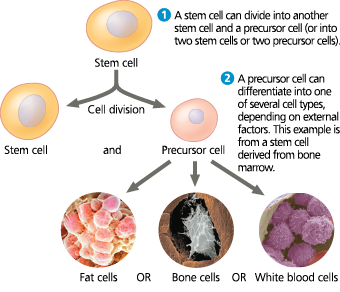
<<Embryonic Stem (ES) Cells:<<
Cells that reproduce indefinitely in culture and can be made to differentiate into a wide variety of specialized cells including eggs and sperm
Working with stem cells - Animal stem cells, which can be isolated from early embryos or adult tissues and grown in culture, are self-perpetuating, relatively undifferentiated cells. Embryonic stem cells are easier to grow than adult stem cells and can theoretically give rise to all types of cells in an organism. The range of cell types that can arise from adult stem cells is not yet fully understood
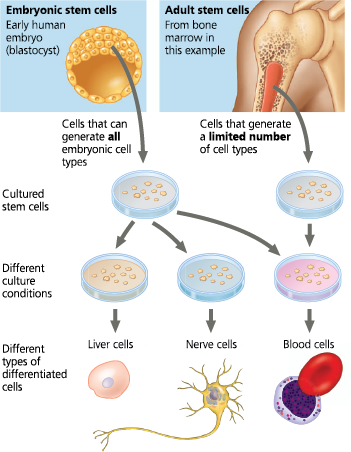
<<Adult Stem Cells:<<
Unable to give rise to all cell types in the organism, though they can generate multiple types
Where are stem cells found?
In bone marrow → blood cells; different bone marrow stem cell → bone, cartilage, fat, muscle, blood vessel linings; adult brain → nerve cells; in skin, hair, eyes, and dental pulp
<<Pluripotent:<<
Describing a cell that can give rise to many, but not all, parts of an organism
<<Therapeutic Cloning:<<
Cloning with the goal to produce ES cells to treat disease
<<Induced Pluripotent Stem (iPS) Cells:<<
Derived from skin or blood cells that have been reprogrammed back into an embryonic-like pluripotent state
A retrovirus was used as a vector to introduce four regulatory genes into fully differentiated human skin fibroblast cells. The cells were then cultured in a medium that would support growth of stem cells 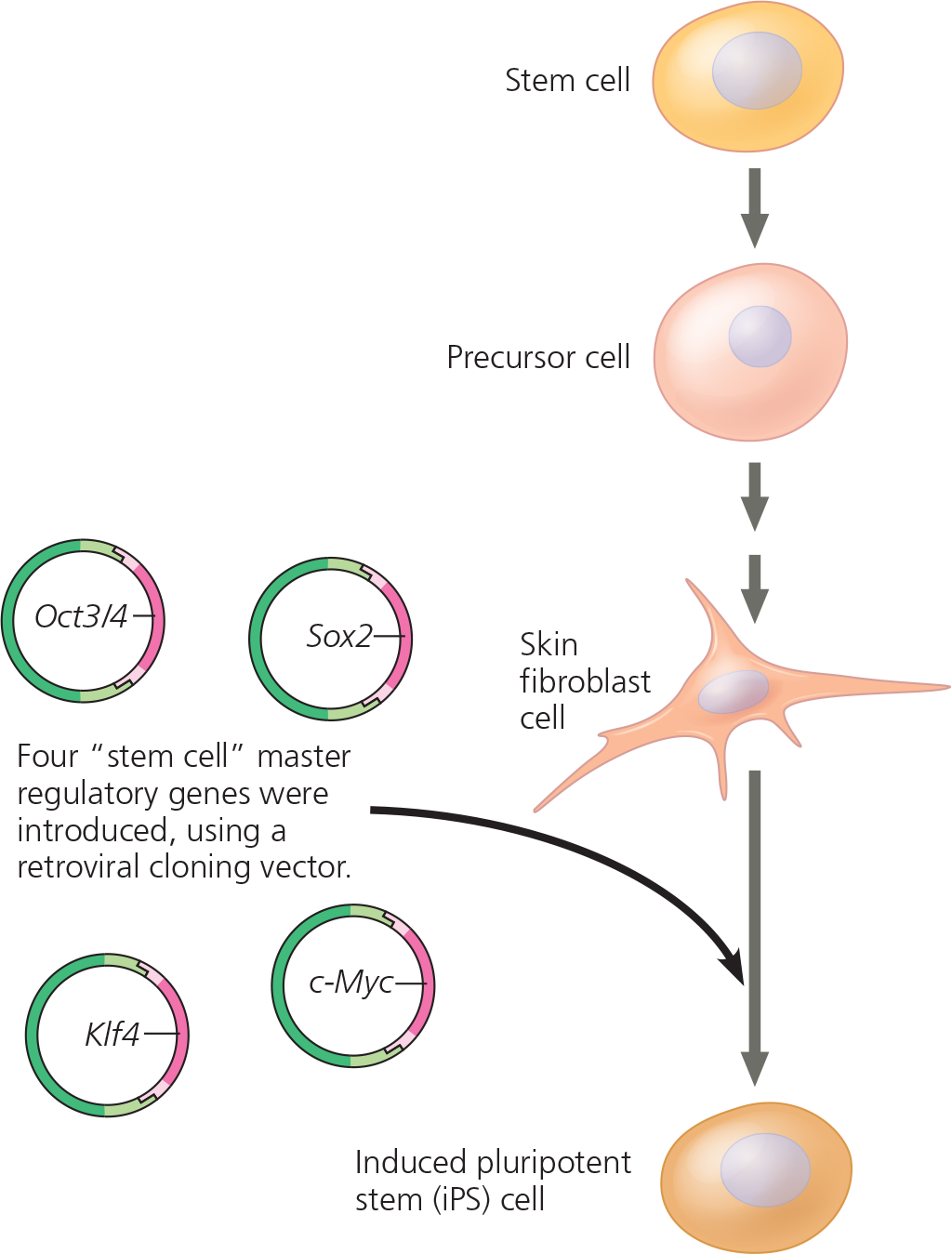
Results - Two weeks later, the cells resembled embryonic stem cells in appearance and were actively dividing. Their gene expression patterns, gene methylation patterns, and other characteristics were also consistent with those of embryonic stem cells. The iPS cells were able to differentiate into heart muscle cells, as well as other cell types
What was concluded from the experiment?
The four regulatory genes induced differentiated skin cells to become pluripotent stem cells, with characteristics of embryonic skin cells
%%When organs are transplanted from a donor to a diseased recipient, the recipient’s immune system may reject the transplant, a condition with serious and often fatal consequences. Would the same risk be faced if the patients’ own cells were used in an iPS-based procedure?%%
Using converted iPS cells would not carry the same risk, which is its major advantage. Because the donor cells would come from the patient, they would be perfectly matched. The patient’s immune system would recognize them as “self” cells and would not mount an attack (which is what leads to rejection)
What are the potential uses for human iPS Cells?
Cells from patients suffering from diseases can be reprogrammed to become iPS cells, which can act as model cells for studying the disease and potential treatments. In the field of regenerative medicine, a patient’s cells could be reprogrammed into iPS cells and then used to replace nonfunctional tissues, such as insulin-producing cells of the pancreas or cells of the retina in the eye
%%Based on current knowledge, how would you explain the difference in the percentage of tadpoles that developed from the two kinds of donor nuclei in Figure 16.11?%%
The state of chromatin modification in the nucleus from the intestinal cell was undoubtedly less similar to that of a nucleus from a fertilized egg, explaining why many fewer of these nuclei were able to be reprogrammed. In contrast, the chromatin in a nucleus from a cell at the four-cell stage would have been much more like that of a nucleus in a fertilized egg and therefore much more easily programmed to direct development.
%%Dolly’s egg donor and surrogate mother were “Scottish blackface” sheep, while the donor of the nucleus was a white-faced sheep. Explain why Dolly has a white face%%
Dolly’s face matched that of the donor of the nucleus, because the nucleus contained the genetic information that determined Dolly’s phenotype. No genetic information was contributed by either the egg donor (the egg was enucleated) or the surrogate mother
%%If you were a doctor who wanted to use iPS cells to treat a patient with severe type 1 diabetes, what new technique would have to be developed?%%
A technique would have to be worked out for turning a human iPS cell into a pancreatic cell (probably by inducing expression of pancreas-specific regulatory genes in the cell)
%%Describe how a researcher could carry out organismal cloning, production of ES cells, and generation of iPS cells, focusing on how the cells are reprogrammed and using mice as an example. (The procedures are basically the same in humans and mice.)%%
Cloning a mouse involves transplanting a nucleus from a differentiated mouse cell into a mouse egg cell that has had its own nucleus removed. Activating the egg cell and promoting its development into an embryo in a surrogate mother results in a mouse that is genetically identical to the mouse that donated the nucleus. In this case, the differentiated nucleus has been reprogrammed by factors in the egg cytoplasm. Mouse ES cells are generated from inner cells in mouse blastocysts, so in this case the cells are “naturally” reprogrammed by the process of reproduction and development. (Cloned mouse embryos can also be used as a source of ES cells.) iPS cells can be generated without the use of embryos from a differentiated adult mouse cell by adding certain transcription factors into the cell. In this case, the transcription factors are reprogramming the cells to become pluripotent
%%16.3 - Abnormal regulation of genes that affect the cell cycle can lead to cancer%%
The products of proto-oncogenes and tumor-suppressor genes control cell division. A DNA change that makes a proto-oncogene excessively active converts it to an oncogene, which then may promote excessive cell division and cancer. A tumor-suppressor gene encodes a protein that inhibits abnormal cell division. A mutation that reduces activity of its protein product may lead to excessive cell division and cancer
Many proto-oncogenes and tumor-suppressor genes encode components of growth-stimulating and growth-inhibiting signaling pathways, respectively, and mutations in these genes can interfere with normal cell-signaling pathways. A hyperactive version of a protein in a stimulatory pathway, such as Ras (a G protein), functions as an oncogene protein. A defective version of a protein in an inhibitory pathway, such as p53 (a transcription activator), fails to function as a tumor suppressor
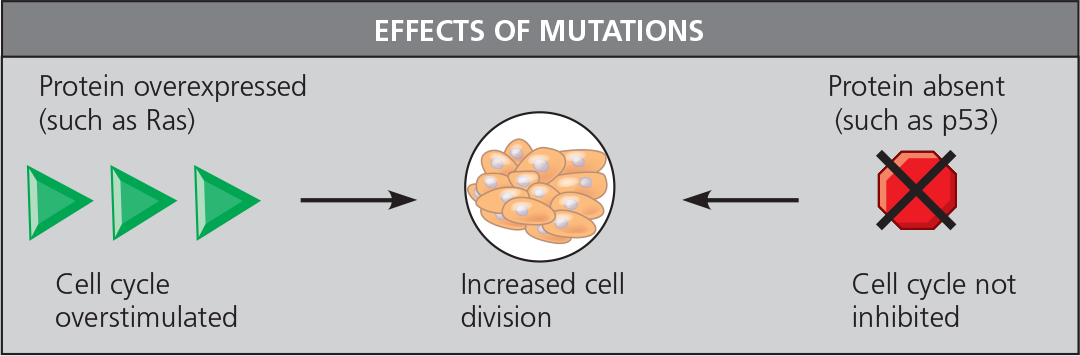
In the multistep model of cancer development, normal cells are converted to cancer cells by the accumulation of mutations affecting proto-oncogenes and tumor-suppressor genes. Technical advances in DNA and mRNA sequencing are enabling cancer treatments that are more individually based
Genomics studies have resulted in four proposed subtypes of breast cancer, based on expression of genes by tumor cells
Individuals who inherit a mutant allele of a proto-oncogene or tumor-suppressor gene have a predisposition to develop a particular cancer. Certain viruses promote cancer by integration of viral DNA into a cell’s genome
The genes that normally regulate cell growth and division during the cell cycle include ___. Mutations that alter any of these genes in somatic cells can lead to _
- genes for growth factors, their receptors, and the intracellular molecules of signaling pathways; cancer
<<Oncogene:<<
A gene found in viral or cellular genomes that is involved in triggering molecular events that can lead to cancer
<<Proto-Oncogene:<<
A normal cellular gene that has the potential to become an oncogene
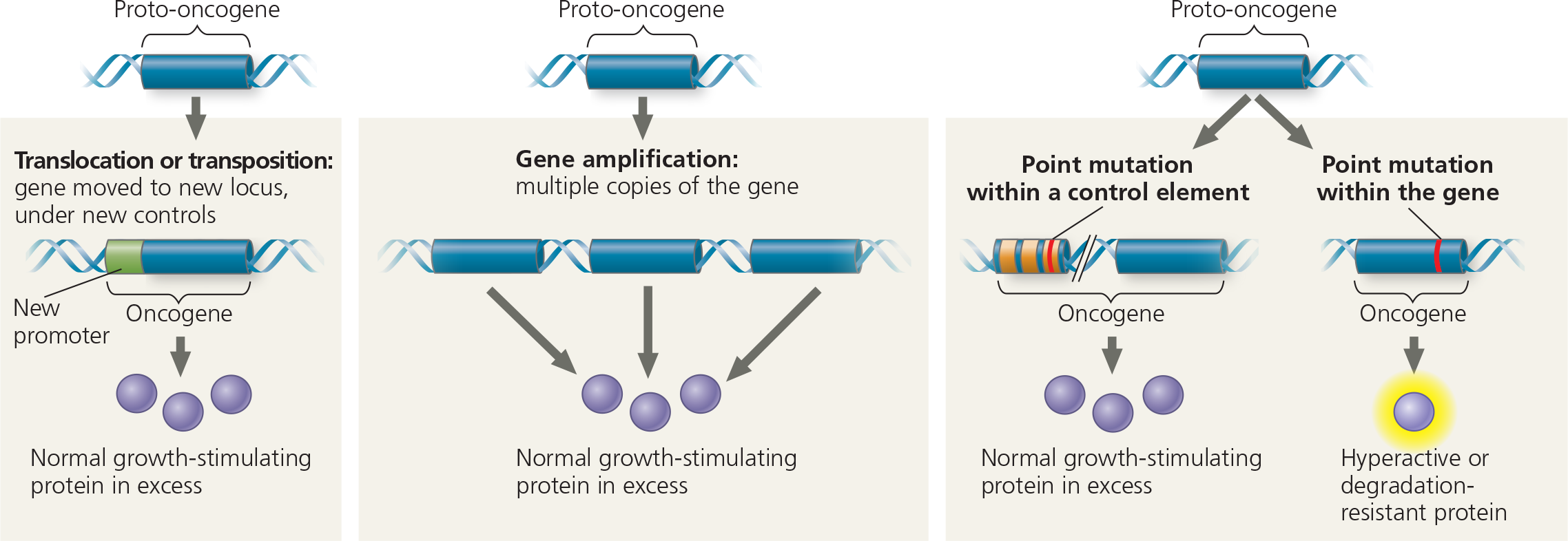
How might a proto-oncogene—a gene that has an essential function in normal cells—become an oncogene, a cancer-causing gene?
In general, an oncogene arises from a genetic change that leads to an increase either in the amount of the proto-oncogene’s protein product or in the intrinsic activity of each protein molecule
Genetic changes that can turn proto-oncogenes into oncogenes - The genetic changes that convert proto-oncogenes to oncogenes fall into three main categories: movement of DNA within the genome, amplification of a proto-oncogene, and point mutations in a control element or in the proto-oncogene itself
<<Tumor-Suppressor Gene:<<
A gene whose protein product inhibits cell division, thereby preventing the uncontrolled cell growth that contributes to cancer
What are the functions of tumor-suppressor genes?
They can repair damaged DNA, control the adhesion of cells to each other or to the extracellular matrix, and have components that inhibit the cell cycle
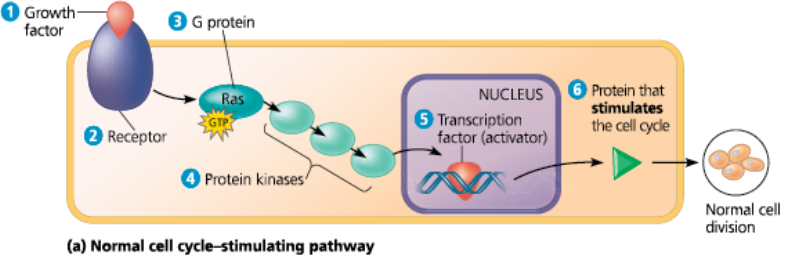
<<Ras Gene:<<
A gene that codes for Ras, a G protein that relays a growth signal from a growth factor receptor on the plasma membrane to a cascade of protein kinases, ultimately resulting in stimulation of the cell cycle
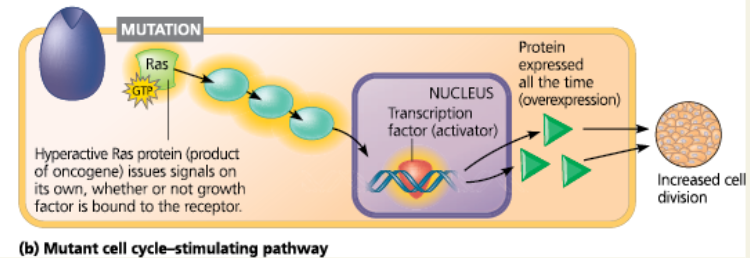
Normal and mutant cell cycle–stimulating pathway - The normal pathway is triggered by 1 a growth factor that binds to 2 its receptor in the plasma membrane. The signal is relayed to 3 a G protein called Ras. Like all G proteins, Ras is active when GTP is bound to it. Ras passes the signal to 4 a series of protein kinases. The last kinase activates 5 a transcription factor (activator) that turns on one or more genes for 6 a protein that stimulates the cell cycle
Normal and mutant cell cycle–stimulating pathway - If a mutation makes Ras or any other pathway component abnormally active, excessive cell division and cancer may result
<<p53 Gene:<<
A tumor-suppressor gene that codes for a specific transcription factor that promotes the synthesis of proteins that inhibit the cell cycle
Normal cell cycle–inhibiting pathway - In the normal pathway, 1 DNA damage is an intracellular signal that is passed via 2 protein kinases, leading to activation of 3 p53. 4 Activated p53 promotes transcription of the gene for 5 a protein that inhibits the cell cycle. The resulting suppression of cell division ensures that the damaged DNA is not replicated. If the DNA damage is irreparable, then the p53 signal leads to apoptosis
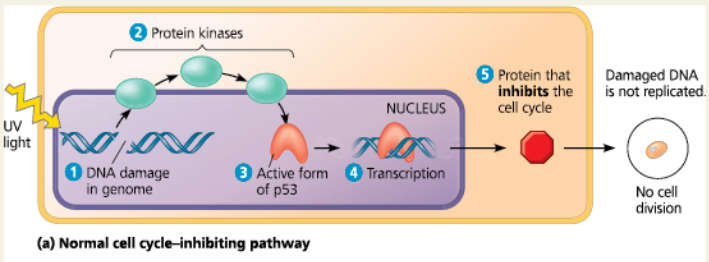
Mutant cell cycle-inhibiting pathway - Mutations causing deficiencies in any pathway component can contribute to the development of cancer
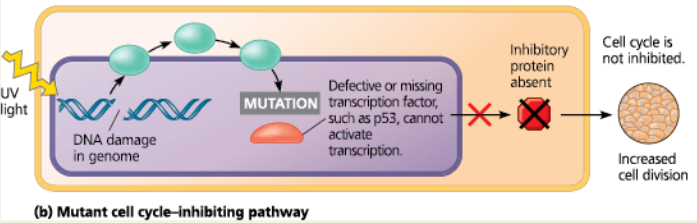
A multistep model for the development of colorectal cancer - This type of cancer is one of the best understood. Changes in a tumor parallel a series of genetic changes, including mutations affecting several tumor-suppressor genes (such as p53) and the ras proto-oncogene. Mutations of tumor-suppressor genes often entail loss (deletion) of the gene. APC stands for “adenomatous polyposis coli,” and SMAD4 is a gene involved in signaling that results in apoptosis
Genomics, Cell Signaling, and Cancer
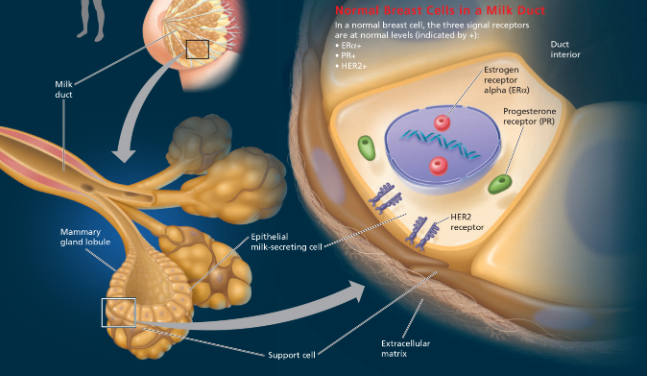
Breast Cancer Subtypes - Luminal A, Luminal B, Basal-like
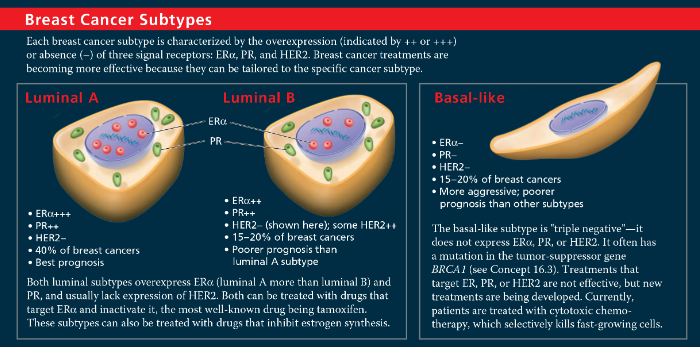
Breast Cancer Subtypes - HER2
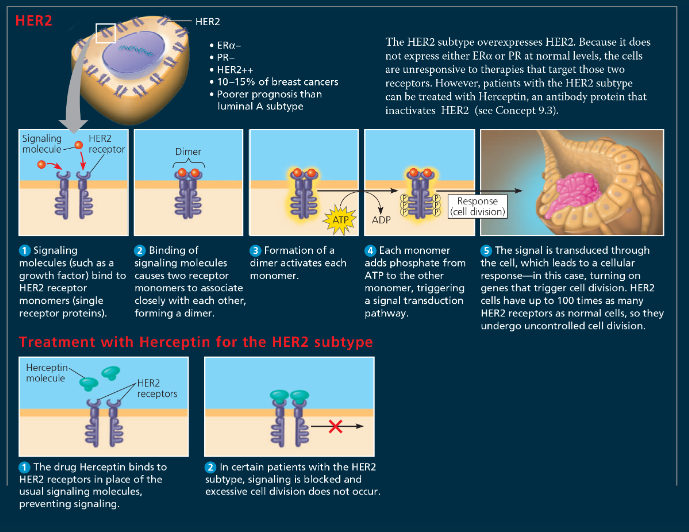
%%When researchers compared gene expression in normal breast cells and cells from breast cancers, they found that the genes showing the most significant differences in expression encoded signal receptors, as shown here. Why does this make sense?%%
Cancer is a disease in which cell division occurs without its usual regulation. Cell division can be stimulated by growth factors that bind to cell-surface receptors. Cancer cells evade these normal controls and can often divide in the absence of growth factors. This suggests that the receptor proteins or some other components in a signaling pathway are abnormal in some way or are expressed at abnormal levels, as seen for the receptors in this figure. Under some circumstances in the mammalian body, steroid hormones such as estrogen and progesterone can also promote cell division. These molecules also use cell-signaling pathways. Because signaling receptors are involved in triggering cells to undergo cell division, it is not surprising that altered genes encoding these proteins might play a significant role in the development of cancer. Genes might be altered either through a mutation that changes the function of the protein product or through a mutation that causes the gene to be expressed at abnormal levels that disrupt the overall regulation of the signaling pathway
<<Adenomatous Polyposis Coli (APC):<<
The tumor-suppressor gene regulates cell migration and adhesion. Mutations of this gene commonly lead to colorectal cancers
<<Tumor Viruses:<<
Capable of directly causing cancer
%%The p53 protein can activate genes involved in apoptosis. Discuss how mutations in genes coding for proteins that function in apoptosis could contribute to cancer%%
Apoptosis is signaled by p53 protein when a cell has extensive DNA damage, so apoptosis plays a protective role in eliminating a cell that might contribute to cancer. If mutations in the genes in the apoptotic pathway blocked apoptosis, a cell with such damage could continue to divide and might lead to tumor formation
%%Under what circumstances is cancer considered to have a hereditary component?%%
When an individual has inherited an oncogene or a mutant allele of a tumor-suppressor gene
%%Cancer-promoting mutations are likely to have different effects on the activities of proteins encoded by proto-oncogenes compared with proteins encoded by tumor-suppressor genes. Explain%%
A cancer-causing mutation in a proto-oncogene usually makes the gene product overactive, whereas a cancer-causing mutation in a tumor-suppressor gene usually makes the gene product nonfunctional
%%Compare the usual functions of proteins encoded by proto-oncogenes with the functions of proteins encoded by tumor-suppressor genes%%
The protein product of a proto-oncogene is usually involved in a pathway that stimulates cell division. The protein product of a tumor-suppressor gene is usually involved in a pathway that inhibits cell division