Chemistry HL S2 (2.1-2.4)
S2
S2.1: The Ionic Model
giving and taking of electrons to form electrostatic attraction
positive ion → cation, negative ion → anion
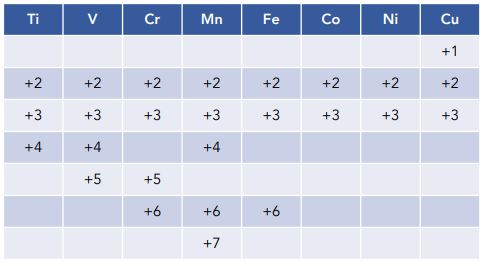
Transition Metals
When the ionization energies for the 1st, 2nd, 3rd, etc. are close to each other, the element will be able to lose those valence electrons easily
results in variable oxidation states depending on the number of valence electrons in the d and s shells
Compound Names
polyatomic ions
nitrate: NO3-
sulfate: SO4²-
phosphate: PO4³-
hydroxide: OH-
hydrocarbonate: HCO3-
carbonate: CO3²-
ammonium: NH4+
Ionic structures and properties
giant ionic lattice structures
made up of very strong electrostatic forces of attraction
ionic bonds or lattice enthalpy
physical properties depend on lattice structures
Lattice Enthalpy (LE)
energy needed to seperate into constituent ions (hence △H>0)
△H is a negative enthalpy change
LE values are a function of the ionic radii and charge
△H = (Knm)/(Rm+ + Rx-)
K - constant
n, m - magnitude of charges
Rm+, Rx- - ionic radii
increase in ionic charge = increase in ionic attraction between ions = increase in lattice enthalpy
increase in ionic radius of on of the ions = decreased attraction between ions = decreased lattice enthalpy
lattice enthalpy is greater for ions with a larger charge density as they have a small radius and are highly charged
Properties of ionic compounds
high melting + boiling point
due to high LE
generally soluble in water (polar) but not in non-polar liquids
good electrical conductivity in liquid/aqueous states
generally brittle
due to crystalline structure
low volatitilty
volatility - tendency of a substance to vaporize
Ionic character and electronegativity
ionic character is determined by the formula:
%ionic character = △Xp/3.2
△Xp=electronegativity difference
bonding continuum
Ionic → △Xp>1.8
Polar Covalent → 0<△Xp<1.8
Covalent → △Xp<1.8
S2.2: The Covalent Model
atoms sharing electrons
Octet Rule
tendency of atoms to gain a valence shell consisting of 8 electrons
pairs of electrons not involved in the bond are called lone pairs
ability of two atoms to form a covalent bond is due to similar strength with which they attract valence electrons
exceptions to the rule
applies to small atoms with less than 8 electrons
forms an incomplete octet
ex: BeCl2, BF3
Bond Strength
bond length → distance between 2 bonded nuclei
bond strength = bond enthalpy → energy required to break the bond
as bond length increases, bond enthalpy decreases
Coordination Bond
bond that is formed by both electrons in pair originating from the same atom
ex: H3O+
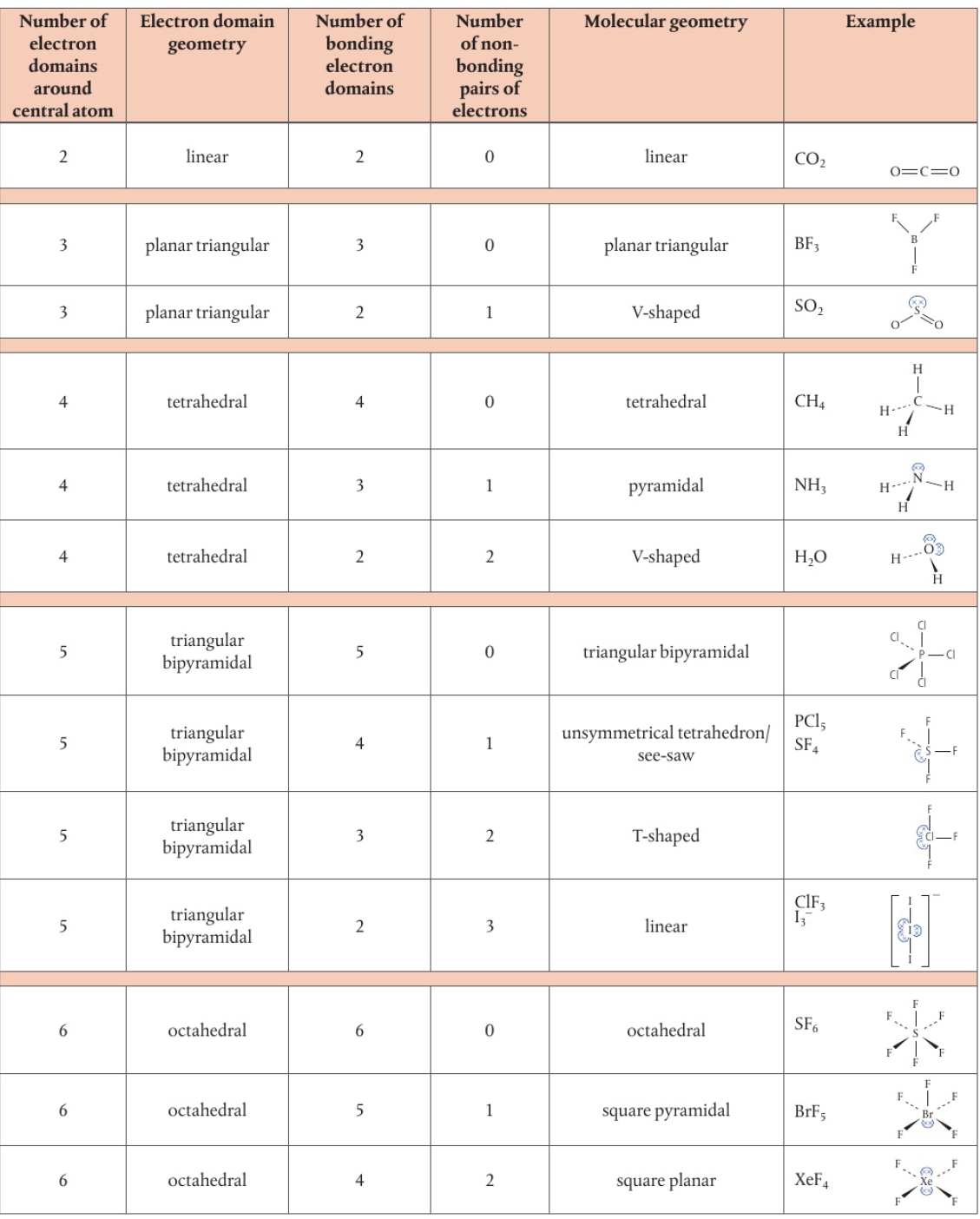
Valence Shell Electron Pair Repulsion (VSEPR)
because electron pairs in the same valence shell carry the same charge, they repel each other and so spread themselves as far as possible
electron pair = electron domain
all electron locations in valence shell, all single, double, triple = 1 electron domain
repulsion applies to electron domains (can be single/double/triple or non-bonding pairs)
total number of electron domains around central atom determines geometrical arrangement of electron domains
shape of molecules is determined by angles between bonded atoms
lone pairs and multiple bonds cause slightly more repulsion
lone pairs have a higher concentration of charge (not shared)
multiple bonds have a higher concentration of charge (more electrons)
non-bonding/lone pair > multiple bond > single bond
Structure
two electron domains
linear shape (bond angle of 180°)
three electron domains
triangular planar (bond angle of 120°) → all single bonds
bent/V-shaped (120°, 121°, 118°) → one double bond
bent/V-shaped (117° bond angle) → one double bond, one lone pair
four electron domains
tetrahedral (109.5°) → all single bonds
trigonal pyramid (107°) → one lone pair
bent/V-shaped (104.5°) → two lone pairs
Bond Polarity
polar bonds - differing electronegativities
different pulling strength for electrons
the more electronegative atom exerts a greater pulling power on the shared electrons → gains more “possesion”
bond dipole - two partially seperated opposite electric charges
the more electronegative atom becomes partially negative
the less electronegative atom becomes partially positive
increased electronegativity difference = increased bond polarity
pure covalent bonds → zero electronegativity difference
polar bonds introduce some ionic nature to the covalent bonds
Pure Covalent
equal sharing of electrons
discrete molecules
Polar Covalent
partial/unequal sharing/transfer of electrons
Ionic
complete transfer of electrons
lattice of oppositely charged ions
Molecular Polarity
depends on polar bonds it contains
depends on molecular geometry
non-polar
dipoles can cancel out, creating non-polar molecules
polar
if the molecule contains bonds of different polarity or the bonds are not symmetrically arranged, dipoles will not cancel out
creates a net dipole (turning force in electric field)
IR active
happens only when an overall dipole moment related to the position and vibration of its bonds is found
Covalent Network Structures
discrete molecules → finite amount of atoms
covalent networks → no finite number of atoms
single repeating pattern of covalent bonds
allotropes → different bonding/structural patterns of the same element in the same physical state, causing different chemical and physical properties
Allotropes of Carbon
Diamond
structure: sp3 hybridied and covalently bonded to 4 others tetrahedrally arranged in a regular repetitive pattern (angle → 109.5°)
non-conductor of electicity, all electrons are bonded
very efficient thermal conducter, better than metals
highly transparent, lustrous crystal
hardest natural substance, brittle, very high melting point
uses: polished for jewelry, tools and machinery for grinding/cutting glass
Graphite
structure: sp2 hybridized and covalently bonded to 3 others, forming hexagons in parallel angles with bond angles of 120° (weak London dispersion forces)
good electrical conductor; contains one delocalized electron per atom
not a good thermal conductor, unless the heat conducts parallel to crystal layers
non-lustrous, grey crystalline solid
soft and slippery due to sliding layers, brittle, very high melting point, stable mostly
uses: dry lubricant, pencils, electrode rods in electrolysis
Graphene
sp2 hybridized and covalently bonded to 3 others, forming hexagons with bond angles of 120° (single layer → 2D only) honeycomb/chicken wire
very good electrical conductor, one delocalized electron per atom
best thermal conductivity known
almost completely transperent
thickness of just one atom (2D) → thinnest material to ever exist, 100x stronger than steel (strongest), very flexible, very high melting point
uses: transmission electron microscopy (TEM) grids, photovoltaic cells, touchscreens, high performance electronic devices, etc.
Fullerene (C60)
sp2 hybridized, bonded in a sphere of 60 carbon atoms, consisting of 12 pentagons and 20 hexagons (closed spherical cage)
poor conductors of electricity, delocalized electron has little movement
very low thermal conductivity
black powder
very light and strong, reacts with potassium (K) to make superconducting crystalline material, low melting point
uses: lubricants, medical, industrial devices for binding specific target molecules; reltaed forms are used to make nanotubes/nanobuds used as capacitators in the electronics indsutry, and catalysts
London Dispersion Forces
non-polar molecules do not have a permanent dipole
electrons behave somewhat like clouds of negative charge, density of the cloud could be greater over one atom at any moment
when there is a differing density, the bond will have seperation of charge, creating a weak dipole (temporary/instantaneous dipole)
creates weak forces of attraction that occur between opposite ends of two temporary dipoles
weakest form of intermolecular force
strength increases with molecular size (greater number of electrons)
LDF is the only force that exists for non-polar molecules
also exists in polar molecules, but is often overlooked for stronger forces
Dipole-dipole attraction
polar molecules have permanent seperation of charge (electronegativity difference)
known as a permanent dipole
opposite charges on neighbouring molecules attracting each other
strength varies on distance and relative orientation of the dipoles
Dipole-induced dipole attraction
occurs in mixtures with both polar and non-polar molecules
the permanent dipole from a polar molecule creates a temporary seperation of charge in the non-polar
act in addition to LDF (non-polar) and dipole-dipole attraction (polar)
van der Waal’s force: all 3 forces added together
Hydrogen Bonding
when a molecule contains hydrogen covalently bonded to fluorine, oxygen, or nitrogen (electronegative atoms)
particular case of dipole-dipole attraction
the large electronegativity difference between hydrogen and the bonded atoms results in the electron pair being pulled away from hydrogen
hydrogen now exerts a strong attractive force on a lone pair in the electronegative atom due to its small size and the absence of other electrons to shield the nucleus
strongest form of intermolecular force
Melting and Boiling Point
changing state = breaking intermolecular forces
covalent substances generally have lower MP and BP than ionic substances
relatively weak intermolecular forces < electrostatic attraction
covalent substances are generally liquid/gas at room temperature
strength of intermolecular forces increase with molecular size and extent of polarity
Solubility
non-polar substances are generally dissolvable in non-polar solvents by formation of LDF between solute and solvent
polar covalent compounds can generally dissolve in water (highly polar H2O) through dipole interactions and hydrogen bonding
solubility of polar compounds is reduced in larger molecules
polar bonds only a small part of the structure
non-polar parts reduce solubility
inability of non-polar groups to associate with water means non-polar substances do not dissolve well in water
polar substances have low solubility in non-polar solvents
they remain together due to dipole-dipole attractions
giant molecular are generally insoluble in all solvents
too much energy required to break the strong covalent bonds
Electrical Conductivity
covalent compounds do not contain ions, so they cannot conduct electricity in the solid or liquid state
some polar covalent molecules (when they can ionize) will conduct electricity
Resonance Structures
delocalization - tendencty to be shared between more than bonding position
delocalized electrons spread out → greater stability for molecule/ion
delocalization occurs when there is more than one position for a double bond within a molecule
two equally valid positions for a double bond
ex: expected is 1 single and 1 double bond, in reality is is 2 equal bonds, intermediate in length and strength
resonance - molecule is a combination of two Lewis formulas
electrons from the double bond delocalize and spread themselves equally between both possible bonding positions
shown with a dotted line
known as a resonance hybrid
resonance influences bond strengths/lengths which in turn can influence reactivity
Benzene (C6H6)
six carbon atoms arranged in a hexagonal ring, each bonded to a hydrogen atom in a triangular planar arrangement with bond angle 120°
true form of benzene is the resonance hybrid
circle represents equally spread delocalized electrons
1-1 ratio of carbon to hydrogen indicates a high degree of unsaturation, greater than that of alkenes or alkynes
does not show characteristic properties
no isomers, reluctant to undergo additional reactions
Expanded Octet
when the central atom is period 3 or lower, sometimes there are more than 8 electrons around the central atom
d orbitals available in the valence shell have energy values similar to those of the p orbitals
promotion of electrons (3p→3d) allows additional electron pairs to form
causes some elements to expand their octets (5-6 electron domains)
Species with five electron domains
triangular bipyramidal shape → angles of 90° and 120°
if one or more domains are non-bonding electrons, they will repel the most
one lone pair gives an unsymmetrical tetrahedron or see-saw shape (bond angles <120° and <90°)
to minimize additional repulsion and bonding domains, the lone pair must be located in an equatorial position (horizontal plane around central atom) instead of an axial osition (above/below horizontal plane)
two lone pairs give a T-shaped structure (bond angles <90°)
three lone pairs give a linear shape (bond angle 180°)
Species with six electron domains
octahedral shape with angles of 90°
no lone pairs of electrons → symmetrical octahedral shape
one lone pair → square pyramidal shape (bond angles slightly less than 90°)
two lone pairs → square planar shape (bond angles of 90°)
maximizes distance apart by arranging pairs on opposite sides
 Molecular Polarity
Molecular Polarity
Formal Charge
formal charge used to predict a preferred Lewis formula
treats covalent bonds as if they were purely covalent with equal electron distribution
FC = V - (1/2 B + L)
V = valence, B = bonding, L = lone (number of electrons)
low FC means less charge transfer has taken place in forming a structure from its atoms
generally means most stable → preferred structure
sum of formal charges for a species must be equal to the charge
Sigma Bond
when two atomic orbitals combine head-on along the bond axis (imaginary line)
overlap of s, p, and hybrid orbitals in different combinations
always the bond that forms in a single covalent bond
electron density is concentrated between the nuclei of the bonded atoms
Pi Bond
when two p orbitals collide laterally (sideways-on)
electron density is concentrated above and below the plane of the bond axis
only forms within double and triple bonds
weaker than sigma bonds as electron density is further from nucleus
Sigma and Pi Bonds
s+s → sigma
s+p → sigma
p+p (head-on) → sigma
hybrid + s → sigma
hybrid + hybrid → sigma
p+p (laterally) → pi
Hybridization
formation of covalent bonds often starts with excitation of the atoms
amount of energy put in to achieve this is more than compensated by the extra energy released on forming bonds
if different orbitals are used in forming covalent bonds, unequal bonds are expected
instead, unequal atomic orbitals within an atom mix to form new hybrid atomic orbitals which are identical but different from the original bonds
hybrid orbirtals have different energies, shapes, and orientation in space from their parent orbitals
allows them to form stronger bonds by allowing for greater overlap
sp³ orbitals
1 s orbital and 2 p orbitals produce 4 sp³ orbitals
shape and energy have properties of s and p, but more like p than s
sp² orbitals
1 s orbital and 2p orbitals produce 3 sp² orbitals
sp orbitals
1 s orbital and 1 p orbital produce 2 sp orbitals
Carbon → Hybridization
C: atomic nyumber = 6 (1s²2s²2px12py1)
forms 4 covalent bonds, but only has two singly occupied bonding electrons
excitation occurs (2s → 2p) to change from ground state
sp³ hybridization
orbitals orientate themselves at 109.5°, forming a tetrahedron
each hybrid orbital overlaps with an atomic orbital → 4 sigma bonds
sp² hybridization
when carbon forms a double bond
orientate themselves at 120°, forming a triangular planar
each hybrid orbital overlaps with a neigbouring atomic orbital → 3 sigma bonds
as the 2 carbon atoms appproach each other, the p orbitals in each atom that did not hybridize overlap sideways
forms a pi bond
double bond (C2) → 1 sigma, 1 pi
characteristic lobes of electron density above and below the bond axis
sp hybridization
orientate themselves at 180°, giving a linear shape
overlap of the two hybrid orbitals with other atomic orbitals → 2 sigma bonds
when carbon forms a triple bond
C2H2
each carbon atom has 2 unhybridized p orbitals that are orientated 90° to each other
combines to form 2 pi bonds
four lobes of electron density turns into a cylinder of negative charge around the atom, making the molecule susceptible to electrophilic reactants (attracted to electron-dense regions)
Hybridization and Molecular Geometry
tetrahedral → sp³
triangular planar → sp²
linear → sp
lone pairs can also be used in hybridization
non-bonding pairs can also hybridize
ex: NH3 → lone pair in N resides in the sp³ orbital
Hybridization and Benzene (C6H6)
each of the 6 carbon atoms are sp² hybridized
forms 3 sigma bonds (120°) → planar shape
leaves the unhybridized p electron on each carbon atom
dumbbell shape perpendicular to the plane of the ring
do not form pi bonds but effectively overlap in both directions
spreads themselves evenly to be shared by all 6 carbon atoms
forms a delocalized pi electron cloud
electron density is concentrated in 2 donut-shaped rings above and below the plane
2.3: The Metallic Model
Metallic Bonding
metals: low ionization energies so they react by losing valence electrons forming a positive ion
metallic character: loss of control over outer shell electrons
when there is no other element present to accept the electrons and form an ionic compound, the outer electrons are held loosely by the nucleus so they ‘wander off’
delocalized electrons
metal atoms form a regular lattice structure through which electrons move freely
metallic bonding: force of electrostatic attraction between lattice of cations and delocalized electrons
Uses of metals
Good electrical conductivity
because of highly mobile delocalized electrons
used for electrical circuits (copper)
Good thermal conductivity
because of delocalized electrons and closely packed ions
used for pots and pans for cooking
Malleable (can be shaped under pressure)
because of the lack of direction in the movement of delocalized electrons
used for machinery
Ductile (can be drawn out into threads)
because the metallic bond remaining intact while formation changes
used for electric wires and cables
High melting points
because of strong electrostatic forces
used for high-speed tools
Shiny, lustrous appearance
because delocalized electrons in metal crystal structure reflect light
used for jewelry
non-directional nature of metallic bonding allows metals to mix with other metals or non-metals in the molten state
resulting mixture is an alloy
enhances properties of the metallic structure
Metallic bond strength
determined by
number of delocalized electrons
charge of the cation
radius of the cation
the greater the electron density and the smaller the cation, the greater the electrostatic attraction
Across a period
increasing melting point
greater attraction between ions and delocalized electrons
lower degree of reactivity
Down a group
decreasing melting point
weaker attraction between ions and delocalized electrons
higher degree of reactivity
Transition elements
elements with an incomplete d-sublevel OR elements that can give rise to cations with an incomplete d-sublevel
proximity in energy between outer occupied sublevels enables them to delocalize large amounts of d-electrons to form metallic bonds
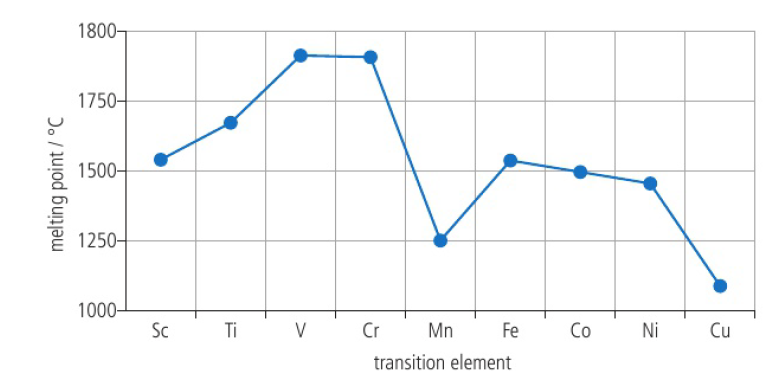
Transition elements: High melting point
metals have a large amount of delocalized electrons and a large positive charge on the metal cations which leads to strong metallic bonding → high melting points
transition metal trends are less evident due to ability of transition elements to delocalize large numbers of electrons and the similar ionic radii
difficult to predict trends accurately compared to the s-block metals
Transition elements: High electrical conductivity
metals have a large amount of delocalized electrons which increases their conductivity
example: copper (Cu) is used in wires
2.4: Models to Materials
Bonding Triangle
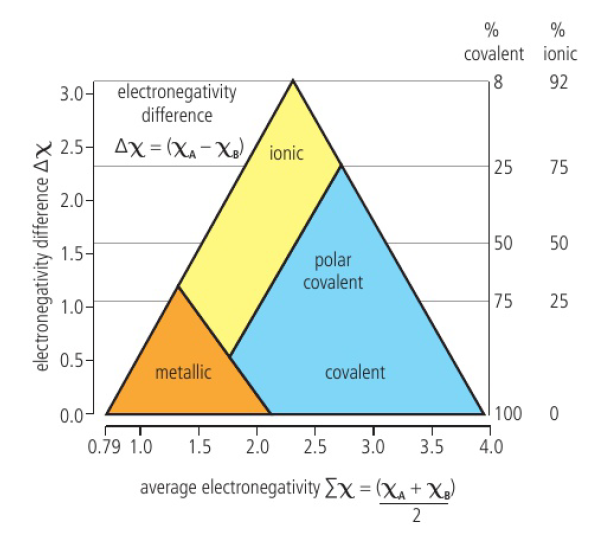
bonding seen as a continuum (ionic, covalent, metallic) → different bonding types are present to different degrees
position on triangle determined from electronegativity
high electronegativity difference = ionic
low electronegativity difference = covalent or metallic
intermediate electronegativity difference = polar covalent
Composite Materials
mixture between two or more different materials
materials have seperate phases (different positions on bonding triangle)
mixture retains properties of individual materials that compose it
example: fibreglass, concrete
Alloys
produced by adding one metal element to another metal (or carbon) in liquid/molten state so the different atoms can mix
in solid, ions of the different metals are scattered through the lattice
forms a structure of uniform composition
metallic bonds are present → delocalized electrons bind the lattice
possible due to the non-directional nature of the delocalized electrons and accomodation of the lattice to different sizes of ions
alloys have properties distinct of component elements (different packing of cations in lattice)
pure metal → regular arrangment of atoms
interrupted in an alloy by different cations
more difficult for atoms to ‘slip over each other’ → stronger
alloy is sronger, more chemically stable, and more resistant to corrosion
Polymers
monomers (small molecules) are able to react together to form a linked chain held together by covalent bonds, forming a polymer
polymers are macromolecules → composed of thousands of atoms and so are relatively large compared with other molecules
nature/properties of a polymer vary with the monomer, length, and amount of branching
structure is shown as a repeating unit with open bonds on each end
natural polymers - found naturally (example: protein, starch, DNA)
synthetic polymers - human-made and non-biodegradable (example: plastics)
Addition Polymers
addition reaction - a multiple bond in a molecule breaks and creates new bonding positions
alkenes/alkynes have double/triple carbon-carbon bonds respectively so they readily undergo addition reactions
they can act as monomers and form addition polymers
%atom economy = molar mass of desired product / molar mass of all reactants x 100
addition polymerization reactions do not generate a by-product so it has a 100% atom economy
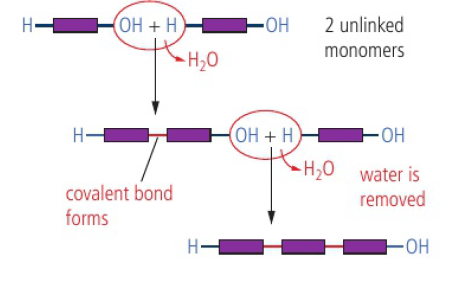
Condensation Polymers
condensation reaction - two functional groups react to form a new covalent bond with the release of a small molecule (H2O, HCl, NH3, etc.)
A-OH + H-B → A-B + H2O
to form condensation polymers, monomers must have functional groups (active ends)
allows them to form new covalent bonds with neighbours on both sides
the functional groups in neighbouring molecules must be able to react together
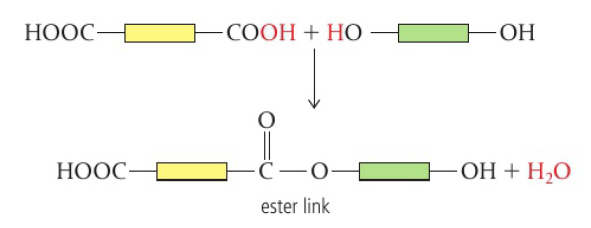
Carboxylic acid + alcohol → polyester (ester link)
when one monomer has two carboxylic acid groups (COOH) and the other has two alcohol groups (OH), an ester link forms between them
chain extends in both directions → polyester
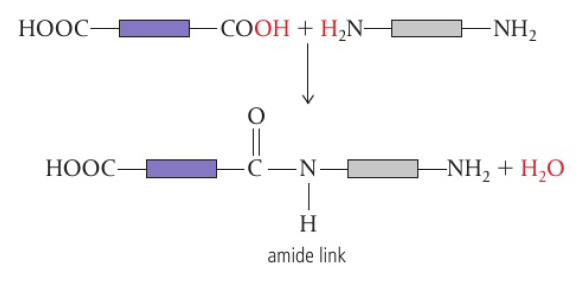
Carboxylic acid + amine → polyamide (amide link)
when one monomer has two carboxylic acid groups (COOH) and another monomer has two amine groups (NH2), an amide link forms between them
forms a polymer known as polyamide