AP BIO UNIT:3(Cellular Energetics)(UPDATED 10/01/24)
Key Vocabulary:
Autotroph: An organism that create it’s own food.
+Photoautotroph: Any organism that produces its own chemical energy by using photosynthesis.
Chemoautotroph: Organisms that derive energy from the oxidation of inorganic compounds(often sulfur compounds).
Heterotroph: Any organism that cannot create its own chemical energy and therefore must acquire it from the environment around it.
ACTIVATION ENERGY:
For any kind of reaction to occur, there must be the involvement of energy. Without energy, a reaction cannot occur. Therefore, the chemical bonds of a cell must be ‘broken’/contorted in some way, shape, or form to provide the reaction to take place.
KEY POINTS OF ACTIVATION ENERGY:
The higher the activation rate, the slower the chemical reaction (Some form of input must be introduced to provide the cell with the energy to make the chemical reaction occur.
ENZYMES AND THE ACTIVE SITE:
An enzyme is the catalyst protein of a cell. The job of an enzyme is to limit the amount of power required for a chemical reaction to take place, this way the cell can utilize this energy for other operations.
KEY POINTS OF ENZYMES:
Enzymes lower the ‘power’(energy) used during the state of transition
ACTIVE SITE:
For the reaction to catalyze, the enzyme(s) must bond to a substrate, a reactant molecule. The substrate(s) bind to the active zone, and therefore play their course in allowing the enzyme to allow a chemical reaction to occur.
KEY POINTS OF AN ACTIVE SITE:
The substrates bind to the enzyme to allow the chemical reaction to occur
The substrate proteins are made of amino acids, containing hydrophilic and hydrophobic chains
ENZYME REGULATION:
Enzymes can also be regulated by different molecules, also known as inhibitors and activators. These molecules have the capability of blocking or promoting the function of the enzyme. This binding is reversible, meaning that the inhibitor/activator is not always attached.
COMPETITIVE AND NON-COMPETITIVE:
Competitive inhibitors fight against the substrate in a ‘competition’ to fit into the active site. The active site has a special form, or mold, that allows the competitive inhibitor to house inside of the active zone.
Non-Competitive inhibitors are capable of being present at the same time as a substrate. Said inhibitor simply attaches itself outside of the active zone to where it is capable of blocking the enzyme from doing it’s job at an allosteric site. While the enzyme can still technically do it’s job, it functions not as quickly as if it were unaffected.
COFACTORS AND COENZYMES:
Without the addition of a beneficiary addition to an enzyme, the enzyme may or may not fully function alone. Oftentimes do cofactors bond to an enzyme through an ionic or hydrogen bond, or with more stability through a covalent bond. The carbon-based aspects of a cofactor are known as coenzymes. These coenzymes are almost always found in vitamins and nutrients.
ATP AND REACTION COUPLING:
Adenosine Triphosphate(ATP) can be thought of as the currency of the cellular world. During the process of the breakdown of ATP(hydrolysis), powers lots of the cell’s actions as it becomes ADP.
DIFFERENT TYPES OF REACTION COUPLING:
Different types of reaction coupling can happen for the different types of reactions that need to happen.
Light (In)Dependent Reactions:
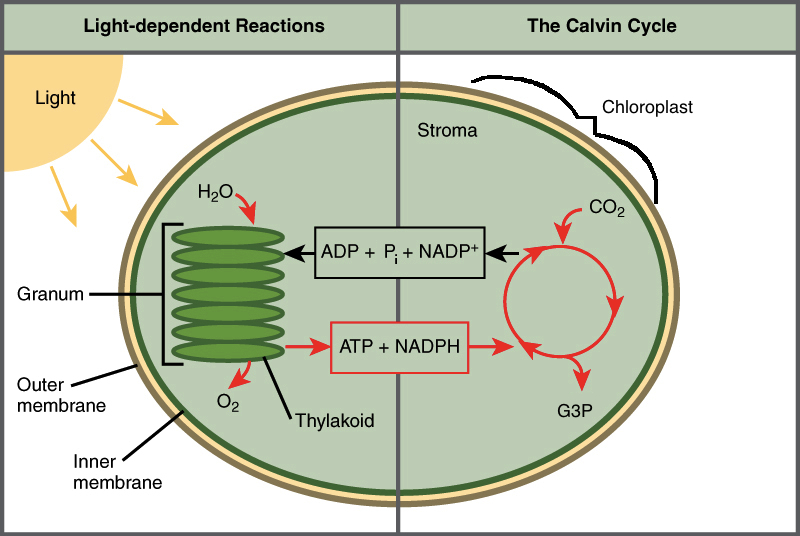
Light dependent reactions are heavily based upon light energy. Light allows for the next two stages of the photosynthesis process. Within plants, the reaction of light occurs within the thylakoid membranes of the organelle chloroplasts.
The Calvin Cycle:
Carbon Dioxide(CO2) is absorbed by the pores of plants and diffuses into the stroma, the jelly-like substance of chloroplasts. Three-carbon surgars are built during the Calvin Cycle, dependent on ATP produced by the light reactions. The reactions of the Calvin Cycle, unlike taking place in the thylakoid membrane, occur within the stroma of the chloroplast.
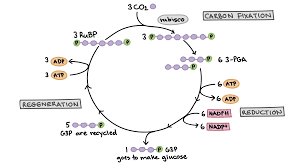
The Calvin cycle reactions can be divided into three main stages: carbon fixation, reduction, and regeneration of the starting molecule.
Carbon Fixation
Reduction
Regeneration
The final result of the Calvin Cycle and the light reactions are to create glucose(‘food’ for the plant). Starch and cellulose are both a form of energy.
The structure of cellulose and starch matters as starch is the stored area of energy. Glucose provides a resource to clean out the body of a human, plant, or animal.
Summary:
Series of enzyme-catalyzed reactions that fix atmospheric carbon in order to produce glucose
Requires investment of ATP and NADPH(high energy electron carrier), meaning these reactions cost energy
ATP and NADPH come from the light reaction, power the production of glucose
Glucose is produced to be used(mostly) for cellular respiration
Introduction to Cellular Respiration and Redox
The process of cellular respiration involves the breaking down of a glucose molecule into carbon dioxide and water. Throughout said process, ATP is produced directly through the transformation of glucose. More ATP is introduced later on within the timeline of cellular respiration through oxidative phosphorylation. Oxidative phosphorylation is fueled by an electron transport chain, a series of protons within the inner membrane of mitochondrion.
During cellular respiration, a glucose molecule is gradually broken down into carbon dioxide and water. Along the way, some ATP is produced directly in the reactions that transform glucose. Much more ATP, however, is produced later in a process called oxidative phosphorylation. Oxidative phosphorylation is powered by the movement of electrons through the electron transport chain, a series of proteins embedded in the inner membrane of the mitochondrion.
Electron carriers, NAD+ and FAD, utilize the electrons originally from the glucose molecules.
Process of ATP:
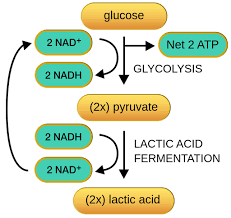
Glycolysis: Within glycolysis, a six-carbon sugar undergoes a series of transformations that converts it into two molecules of pyruvate, a three-carbon organic molecule. In this reaction, ATP is being created and NAD+ is being converted to NADH.
Pyruvate Oxidation: Each of the pyruvates from glycosis are sent to the mitochondrial matrix the innermost area of mitochondrion. A two-carbon molecule bound to Coenzyme A, acetyl, carbon dioxide is released and NADH is created.
Citric Acid Cycle: The acetyl(Coenzyme A) made during pyruvate oxidation combines alongside four-carbon molecules and then is released into multi-stage reactions. ATP is created as well as NADH and FADH2 as carbon dioxide is released.
Oxidative Phosphorylation: The NADH and FADH² deposit electrons upon the electron transport chain and then turn back into their standard forms. As electrons move down the electron chain, the energy of the electrons is released and is utilized to formulate a pump out of the matrix, therefore creating a gradient through chemiosmosis. Water is formulated as oxygen accepts electrons to take protons and therefore form water as a waste product.
Oxidative Phosphorylation(In Essence): The high energy electrons from NADH and FADH² powers the electron transport chain. ATP is created throughout this process of chemiosmosis in ATP synthase. Oxygen is the final electron acceptor of the ETC(electron transport chain), which formulates water as a waste product.
Aerobic Respiration
Anaerobic respiration / fermentation is the production of energy without the consumption of oxygen. This takes two forms, depending on the organism and the conditions it is in.
Lactic Acid Fermentation: Glycosis is converted as normal to become pyruvate. However, after the pyruvate is created, it is then recycled multiple times into lactic acid.
While this may seem like it would happen in only organisms in no relation to humans, it is indeed a process that happens to humans. Lactic acid is received when we, as humans, exercise or are doing strenuous activities.
Alcoholic Fermentation
Glycosis utilizes the glucose to pyruvate process reduces NADH to ethanol and carbon dioxide, both waste products.