AP Biology Midterm
Unit 8
8.1 Responses to an Environment
An organisms behavioral and/or physiological response is related to environmental changes
a stimulus is an external, internal, or combination of signals that causes a resonse from an organism
An organisms behavioral response can affect their overall fitness
Organisms exchange information with one another in response to internal and external signals
communication between organisms can change behavior
known as signaling behavior
Signaling behavior produces changes in the behaviors of other organisms
can result in differential reproductive success
Animals communicate through various mechanisms
Audible
tactical
visual
electrical
communication mechanisms have multiple uses
establish dominance
find food
ensure reproductive success
Response and communication impact natural selection and evolution
Natural selection favors innate and learned behaviors that increase survival and reproductive success
innate behaviors are genetically controlled and can occur without prior experience or training
Learned behaviors are a product of experience
Cooperative disorders increases teamwork between organisms of the same species
increases the fitness of the individual
increases survival of the population
Key takeaways
Organisms respond to changes in their environment to maintain homeostasis, which increases survivability
Organisms exchange information about changes in the environment through various mechanisms
Organisms have a variety of signaling behaviors that can help increase the survival chance of other members in the population, resulting in differential reproductive success
organisms of the same population/environment use signaling mechanisms to alert others of changes in the environment —> increases chances of reproduction
Animals use visual, audible, tactical, electrical, and chemical signals to indicate dominance, find food, establish territory, and ensure reproductive success
Cooperative behavior tends to increase the survival of the individual and increase the fitness of the population
8.2 Energy flow through ecosystems
Organisms use different strategies to regulate body temperature and metabolism
Endotherms use thermal energy generated by metabolism to maintain homeostatic body temperature
Changes in heart rate, fat storage, muscle contraction (shivering)
Echotherms lack efficient internal mechanisms to maintain internal body temperatures
rely on behaviors to regulate temperatures (moving in or out of the sun)
Organisms use energy to grow and reproduce
there is a relationship between metabolic rate per unit of mass and the size of an organism
metabolic rate is the amount of energy expended by an animal over a specific amount of time
a net gain in energy can result in energy storage or growth
A net loss of energy can result in loss of mass and possibly death
generally, the smaller the organism the higher the metabolic rate
Different organisms use various reproductive strategies in response to energy availability
some species produce alot of offspring at one time
less energy efficient
often occurs in unstable environments where the resources are not readily available and the environment experiences frequent change
Some species produce few offspring at one time
more energy efficient
common in stable ecological environments
Changes in energy availability affect populations and ecosystems
changes in energy availability can result in changes in population size
Changes in energy availability can result in disruptions to an ecosystem
a change in energy resources such as sunlight can affect the number and size of trophic levels
a change in the number of producers can affect the number and size of other trophic levels
a trophic level is a position an organism maintains in a food chain
Changes in availability can result in changes in population size
A food chain shows the direction of nutrient transfer from one organism to another
each organism occupies a different tropic level and reflects how many energy transfers came before it
primary producer —> primary consumer —> secondary consumer —> tertiary consumer —> quaternary consumer (apex) —> decomposer
Food webs consist of many interconnected food chains
The transfer of energy between trophic levels is inefficient
typically around 10% efficient
This energy inefficiency limits the length of food chains and the size of populations
typically, population size decreases up trophic levels
The activities of autotrophs and heterotrophs enable the flow of energy within an ecosystem
Autotrophs are organisms that capture energy from physical or chemical sources in the environment
photosynthetic organisms capture energy from sunlight
Chemosynthetic organisms capture energy from small inorganic molecules present in their environment with or without organisms
Heterotrophs capture energy present in carbon compounds produced by other organisms
Metabolize carbohydrates, lipids, and proteins, as sources of energy by hydrolysis
Key Takeaways
Endotherms and ectotherms utilize different mechanisms to regulate body temperature and metabolism
Different organisms use various reproductive strategies in response to energy availability
There is a relationship between the metabolic rate per unit of body mass and the size of multicellular organisms generally the smaller the organism —> the higher the the metabolic rate
A net gain in energy results in energy storage and growth, whereas a net loss in energy results in loss of mass and potentially death
Changes in energy availability can result in changes to population size and disruption to an environment
Heterotrophs capture energy present in carbon compounds produced by other organisms
Autotrophs such as photosynthetic and chemosynthetic organisms capture energy from physical or chemical organisms in an environment
8.3 Population ecology
A population is comprised of individual organisms of the same species
a population is comprised of indvidual organisms of the same species in a particular area
individuals interact with one another in complex ways
individuals in a population usually interbreed with one another of the same species rather than mating with other populations
Many adaptations are related to obtaining and using energy in a particular environment
the size of populations largely depends on available resources
when food is less available, the population size decreases
less food energy is available to support individuals
reproduction rates decrease
offspring survivability decreases
when food is readily available, the population size increases
reproduction rates increase
More food for offspring
Survival rates increase
Different species have adaptations for when energy is not readily available
storage of fat during winter months
losing leaves or growing leaves when the day length changes
migrating in response to changes in food availability
Population growth dynamics depend on a number of factors
several factors can effect population growth
age at reproductive maturity
number of offspring produced
frequency of reproduction
Reproduction with no constraints results in the exponential growth of a population
Exponential growth refers to sharp increase in the growth of a population
occurs under ideal conditions, when resources are abundant
more individuals are reproducing
How long it takes to produce offspring is the same
Exponential growth is represented by a J shaped curve
Key Takeaways
Populations comprise individual organisms of the same species thst interact with one another and with the envornment in complex ways
Population growth dynamics depend on several factors, including time, birth rate, death rate, and population size
Reproduction without constraints results in the exponential growth of a population
8.4 Effect of Density of Populations
Resource availability in an environment impacts population density
Population density refers to how close individuals within a population live near one another
Higher reproductive rate
space is limited
When doos is limited, the density of a population may decrease
Lower reproductive rate
individuals can spread out in the limited space
Limits to population growth are due to density dependent and density independent
Density dependent factors are abiotic or biotic factors whose effect on population size relies on a population’s density
Competition for resources
territoriality
disease
predation
Density independent factors are abiotic or biotic factors that affect population size regardless of population density
Natural disasters
floods
forest fires
volvanic eruptions
pollution
A population can produce a density of individuals that exceeds the systems resource availability
a logistic growth model describes population growth that intially starts slowly, immediately followed by exponential growth, and ends with a relatively stable maximum growth
illustrated in an S shaped curve
Maximum number of individuals an environment can sustain is referred as the carrying capacity
Both density dependent and density independent limiting factors cause a population to reach carrying capacity
Under certain conditions, a population can temporarily exceed the carrying capacity
limiting factors will always bring population size back down
fluctuations in population size can naturally occur at or near carrying capacity
Population dynamics can be represented using mathematical models
Key Takeaways
A population can produce a density of individuals that exceeds the system resource availability
When density dependent and density independent factors are imposed, a logistic growth model generally ensues
8.5 Community Ecology
The structure of a community is described according to species composition and diversity
a community refers to a group of different species living together in the same location and interacting with one another
Communities are described based on species diversities and species composition
species diversity refers to the variety species and the quantity of individuals included in each species within a given community
Species composition refers to the identity of each species in the community
The structure of community is measured in terms of species composition and diversity
species diversity can be measured using an equation called the simpsons diversity index
used to measure the biodiversity of a habitat
the higher the index value, the more diverse the community
based on random samples in the environment
Interactions among populations determine how they access energy and matter
communities change over time depending on interactions between populations
competition is an interaction that can affect how populations access energy and matter
can result in a change in community structure
competition for food an habitats
Can be positive, negative, or neutral
Positive interactions
mutualism : both species benefit
Commensalism : one species benfits but the other is not harmed or helped
Negative interactions
parasitism : one species uses another for a food source
predator prey : one species uses another for a food source
Neutral interactions have no impact on the species involved
Interactions among populations determine how they access energy and matter
bracket or shelf fungi are tree parasites
they produce fruiting bodies that grow on the bark of the tree
the absorb nutrients from the outer bark of the tree
can cause weakening of the external structure of the tree
redued canopy and reduced foliage desnity
frees out resources
Can infect interior parts of the tree
provides new availabile niches and habitats
They provide microhabitats for insects and other organisms
Insects can live in the holes the fungi make in the tree bark
They provide a food source for insects and other organisms
some insects use the fungi as a food source
Relationships among interacting populations can be modeled
Key Takeaways
The structure of a community is measured and described in terms of species composition and species diversity
Communities change over time depdening on interactions between populations
Interactions among populations determine how they acces energy and matter within a community
Relationships among interacting populations can be characterized by positive and negative effects and can be modeled
Competition, predation, and symbioses, including parasitism, and commensalism, can drive population dynamics
8.6 Biodiversity
Ecosystem diversity is related to its resilience to changes in the environment
natural and aritifical ecosystems with fewer component parts and with little diversity among the parts are often less resilient to changes in the environment
the diversity of a species within an ecosystem may influence the organization of the ecosystem
The long term structure of an ecosystem cab be stabilized with more diversity
less vulnerable to drastic structural changes when environment changes or when organisms are added and or removed
Changes in diversity can cause short term and long term structural changes in the community
Abiotic and biotic factors contribute to maintaining the diversity of an ecosystem
the diversity of species within an ecosystem may influence the organization of the system
abiotic factors help maintain system diversity
climate
water and nutrient availability
light availability
Biotic factors help maintain ecosystem diversity
producers help maintain ecosystem diversity
many populations depend on producers for food and habitats
reduce erosion
Dominant predators keep prey populations under control
Have diversified diets
dont put too much pressure on any one population
The effects of keystone species are disproportionate relative to their abundance
keystone species are species the community structure depends on
smaller populations compared to other populations in the community
When they are removed from the ecosystem, the ecosystem often collapses
they often control the size of multiple other populations
Overpopulation depletes resources
Key Takeaways
Natural and artificial ecosystems with fewer component parts and with little diversity among the parts are often less resilient to changes in the environment
The longer term structure of an ecosystem can be stabilized with more diversity. Changes in diversity can cause short term and long term structural changes in a community
Keystone species, producers, and esstial abiotic and biotic factors contribute to maintaining the diversity of an ecosystem
The effects of keystone species on the ecosystem are disproportionate relative to their abundance in the ecosystem, and when thet are removed from the ecosystem, the ecosystem collapses
8.7 Disruptions to Ecosystems
Evolution is characterized by change in the genetic makeup of a population over time
an adaptation is a genetic variation that is favored by selection
is manifested as a trait that provides an advantage to an organism in a particular environment
can arise through mutations
mutations are random and are not directed by specific environmental pressures
Will increase in frequency if environment continues to select for this trait
increases biodiversity
populations evolve
specitation can occur
Invasive species affect ecosystem dynamics
the availability of resources can result in uncontrolled population growth and ecological changes
an invasive species is one that is not native to a specific area and harms the community it is introduced to
the introduction of an invasice species can be intentional or unintentional
invasive species exploit new niches
nich is free of predators or competitors
outcompete other organisms for resources
population increases unchecked
The distribution of local and global ecosystems changes over time
habitat change can occur because of human activity
human impact accelerates change at local and global levels
urbanization
deforestation
erosion
extinction
pollution
climate change
The introduction of new diseases can devastate native species
human migration and overpopulation can accelerate spread of disease
Geological and meterological activites lead to changes in ecosystems
geological and meteorological events affect habitat change and ecosystem distribution
large habitat disruptions
chemical disruptions
Can accelerate evolution
reproductive isolation
change in selective advantage
new niches
Can Cause extinction
Biogeographical studeis illustrate these changes
analyze species disitribution
can be used to characterize biomes
Key Takeaways
An adaptation is a genetic variation that is favored by selection and is manifested as a trait that provides an advantage to an organism in a particular environment
The intentional or unintentional introduction of an invasive species can allow the species to exploit a new niche free of predators or competitors or to outcompete other organisms for resources
The introduction of new diseases can devastate native species. Habitat change can occur because of human activity
Geological and meteorological events affect habitate change and ecosystem distribution
Unit 1
1.1 Structure of Water and Hydrogen Bonding
The subcomponents of biological molecules determine the properties of that molecule
Water is composed of 2 main elements, oxygen and hydrogen, in a 1:2 ratio respectively
Covalent bon is the term used to describe the bond type in which atoms share electrons
oxygen is more elctronegative compared to hydrogen, resulting in an unequal sharing of electrons between oxygen and hydrogen
Covalent bonding can result in plarity when there are differences in atomic electronegativities, a water molecule has polarity
A hydrogen bond is a weak bond interaction between the negative and positive regions of two separate molecules
Water can form hydrogen bonds with other water molecules or with other charged molecules
When two of the same molecules form hydrogen bonds with eachother this is called cohesion
When two different molecules form hydrogen bonds with eachother this is called adhesion
Living systems deoend upon properties of water
the hydrogen bonds between water molecules can result in surface tension
cohesion, adhesion, and surface tension allow for water to demonstate additional chemical behaviors known as emergent properties
Key Takeaways
Water contains 1 oxygen atom covalently bonded to 2 hydrogen atoms
Oxygen has a higher electronegativity compared to hydrogen resulting in a water molecule having polarity
Polarity allows molecules to form hydrogen bonds when oopositely charged regions of two molecules interact
The term cohesion refers to molecules of the same type forming hydrogen bonds with one another and adhesion refers to different types of molecules forming hydrogen bonds with one another
Living systems depend upon waters properties, like surface tension
1.2 Elements of life
Living systems require a constant input of energy
the law of conservation of energy states that energy cannot be created or destroyed only transformed
Living systems follow the laws of energy
Living systems need a constant input of energy to grow, reproduce, and maintain organization
Living systems mainly use the energy stored in chemical bonds
Living systems require an exchange of matter
atoms and molecules from the environment are necessary to build new molecules
carbon is used to build bilogical molecules such as carbohydrates, proteins, nucleic acids, and lipids
nitrogen is sued to build proteins and nucleic acids
Phosphorus is used to build nucleic acids and certain lipids
Carbon is used to build macromolecules
caron can bond to other carbon atoms creating carbon skeletons to which other atoms attatch
carbon skeletons allow for the creation of very large and complex molecules
carbon containing molecules can be used to store energy
carbon contsining molecules can be used to form basic cell structures
Key Takeaways
living systems need a constant input of energy to grow, reproduce, and maintain organization
atoms and molecules from the environment are necessary to build new molecules
Carbon is used to build all macromolecules, store energy, and form cells
Nitrogen is used to build proteins and nucleic acids
Phosphorus is used to build nucleic acids and certain lipids
1.3 Introduction to biological macromolecules
Monomers have important properties
monomers are chemical subunits used to create polymers
polymer is a macromolecule made of many monomers
a covalent bond is formed between two interacting monomers
monomers have specific chemical properties that allow them to interact with one another
polymers are specific to the monomers they consist of
monosaccharide is the monomer of carbohydrates
amino acids are monomers of proteins
nucleotides are monomers of nucleic acids
fatty acids are monomers of lipids
Dehydration synthesis reactions form covalent bonds
dehydration synthesis reactions are used to create macromolecules
the subcomponents of a water molecule are removed from interacting monomers and a covalent bond forms between them
The H and OH join together to form a molecule of water, water is a byproduct of this reaction
Hydrolysis reactions cleave covalent bonds
polymers are hydrolyzed into monomers during a hydrolysis reaction
covalent bonds between the monomers are cleaved during a hydrolysis reaction
A water molecules is hydrolyzed into subcomponents and each subcomponent is added to a different monomer
Dehydration synthesis creates carbohydrates
carbohydrate monomers have hydroxides and hydrogen atoms attatched
one monomer will lose an entire hydroxide while the other monomer will only lose a hydrogen from a hydroxide
A covalent bond will form where the hydroxide and hydrogen atom were removed
The hydroxide (OH) and hydrogen (H) join forming a water molecules (H2O)
A dehydration synthesis creates proteins
protein monomers are called amino acids
each amino acid has an amino group (NH2) terminus and a carboxyl group (COOH) terminus
a hydroxide (OH) is lost from the carboxyl group of one amino acid and a hyrdrogen atom is lost from the amino group of another amino acid
a covalent bond will form between the monomers in the location where the hydroxide and hydrogen atom were removed
The hydroxide and hydrogen atom will join forming a water molecule
Proteins can undergo hydrolysis reactions
covalent bonds between amino acids can be cleaved
a water molecle is hydrolyzed and each subcomponent of water will be bonded to different amino acids
the result is separate amino acid molecules
Key Takeaways
all monomers contain carbon and are used to build biological macromolecules
covalent bonds are used to connect monomers together
dehyrdation synthesis reactions are used to create biological macromolecules and water is an additional product
Hydrolysis reactions use water to break down biological macromolecules
1.4 Properties of biological molecules
Function is related to structure
living system are organized in a hierarchy of structural levels
at every level of organization, function is related to structure
A change in structure generally results in a change in function
in living systems the proterites of biological molecyles are determined by the structure and function of the molecules
The structure of Nucleic acids determine functions
nucleic acids are polymers comprised of monomers called nucleotides
Nucleotides have a basic structure that contains 3 main subcomponents : a five carbon sugar, a phosphate group, and a nitrogen base
All nucleic acids store biologcial information in the sequence of nucleotide monomers
There are differences in nucleic acid structure
DNA and RNA are examples of nucleic acids
DNA and RNA nucleotides differ in the type of sugar contained (deoxyribose and ribose)
DNA and RNA nucleotides can differ in the nitrogen base contained (adenine, guaning, thymine, cytosine, uracil)
Although both DNA and RNA store biological information, the structural differences between them result in specific functional differences
Proteins have different structures and functions
amino acids are the monomers that make up proteins
Amino acids have directionality with an amino terminus and a carboxyl terminus
a polypeptide, the primary structure of a protein, consists of a specific order of amino acids and determines the overall shape the protein can achieve
The chemical properties of R groups varies
amino acids differ in the R group, the atoms attatched to the central carbon
The r group can be hydrophobic, hyrdrophyllic, or ionic
A protein can have different amino acids in the polypeptide allowing the protein to have regional differences in structure and function
Carbohydrates and lipids vary in structure and function
complex carbohydrates can have monomers whose structures determine the properties and functions of the carbohydrate
Lipids are nonpolar macromolecules that do not have true monomers but are comprised of subunits such as fatty acids and gylcerol
Lipids have fatty acid components that determine structure and function based on saturation
Specialized lipids, called phospholipids, contain hydrophilic and hydrophobic regions that determine their interactions with other molecules
Membranes contain lipids and proteins
phospholipids and proteins are two main molecules that make up biological membranes
Phospholipids and some membrane proteins have hydrophilic and hyrdrophobic regions
The hydrophilic regions of phospholipids and proteins can interact with eachother and the water environments
The hydrophobic regions of phospholipids and membrane proteins can interact with eachother but cannot interact with water environments
Key Takeaways
Nucleotides can vary in the sugar and base components resulting in nucleic acids with different structure and function
The amino terminus and carboxyl terminus give amino acids directionality and determine how amino acids assemble into protein polymers
R group properties determine how amino acids interact within the polypeptide and determine the structure and function of the protein
Differences in the components of carbohydrate monomers determine how the monomers assemple into complex carbohydrates and determine function
Lipids are nonpolar macromolecules and difference in saturation determine the structure and function of lipids
Phospholipids contain polar regions that interact with other polar molecules and nonpolar regions
1.5 Structure and Function of Biological Macromolecules
Directionality of the subcomponents influences structure of nucleic acid polymers
the linear structure of all nucleic acids is characterized by a 3’ hydroxyl and 5’ phosphate of the sugar in the nucleotide
DNA is a nucleic acid polymer containing two strands, each strand in an antiparallel 5’-3’ direction
Adenine - Thymine base pairs are held together by 2 hydrogen bonds and guanine - cytosine base pairs are held together by 3 hydrogen bonds
hydrogen bonds between base pairs in a DNA molecule stabilize the molecules structure
The linear sequence of nucleotides encodes biological information
Any change to the sequence of the nucleotides may lead to a change in the encoded information
Directionality influences the synthesis of nucleic acids
During the synthesis of nucleic acid polymers, nucleotides can only be added to the 3’ end
Covalent bonds are used to connect free nucleotides to the strand
Key Takeaway
The linear sequences of all nucleic acids is defined by the 3’ hydroxyl and 5’ phosphate of the sugar in the nucleotide
DNA is structured as an antiparallel double helic with two strands running in opposite 5’-3’ directions. This allows for the two strands of DNA to be held together by hydrogen bonds between the base pairs. A-T held together by 2 hydrogen bonds, G-C held together by 3 hydrogen bonds
During DNA and RNA synthesis, nucleotides can only be covalently added to the 3’ end of a growing nucleotide strand
Changes in the linear structure of the nucleotide bases may lead to differences in the encoded biological information or the structural stability of the molecule
Directionality of the subcomponents influences structure of proteins
proteins comprise linear chains of amino acids that have directionality with an amino terminus and carboxyl terminus
Covalent bonds are connected by the formation of covalent bonds at the carboxyl terminus of the growing peptide chain
PRIMARY STRUCTURE is determined by the sequence of amino acids held together by covalent bonds, called peptide bonds
SECONDARY STRUCTURE arises through local folding of the amino acid chain into elements such as alpha helices and beta pleated sheets
TERTIARY STRUCTURE is the overall 3d shape of the protein and often minimizes free energy ; various types of bonds and interactions stabilize the proteins at this level
QUATERNARY STRUCTURE arises from the interactions between multiple polypeptide chains
Four elements of protein structure
primary
secondary
alpha helices
beta pleated sheets
Tertiary
3d functional form for most proteins
Quaternary
Key Takeaways
amino acids have directionality with an amino terminus and carboxyl terminus on the other. Amino acids are added to the carboxyl terminus of a growing peptide chain by the formation of covalent bonds
There are 4 elements of protein structure : primary, secondary (alpha helices, beta pleated sheets), tertiary, quaternary. Levels of structure beyond primary linear sequence of amino acids arise through local folding and other chemical interactions among amino acids. The resulting 3d shape gives rise to the proteins specific functions.
A change in amino acid subunit at the primary level of structure may lead to a change in the structure and function of the protein.
Directionality of the subcomponents influences structure and function of carbohydrates
carbohydrates comprise linear chains of sugar monomers connected by covalent bonds
small direction change in the components of a molecules can result in functional differences
carbohydrate polymers may linear or branched
starch and glycogen both function in energy storage (starch in plants glycogen in humans and other vertebrates)
Cellulose functions as support and provides strength in plant cell walls
Key Takeaways
carbohydrate comprise linear chains of sugar monomers connected by covalent bonds. Sugar monomers may vary in the direction of some their components, such as the bond orientation of OH groups linked to carbon chain
Depending on the type of sugar monomer used in its formation, a carbohydrate polymer may have a linear or branched structure and can differ in function
1.6 Nucleic Acids
Similarities between DNA and RNA
both are assembled from a nucleotide subunits which are comprised of a
5 carbon sugar
phosphate group
nitrogenous base
Each nucleotide monomer is connected by covalent bonds forming the sugar phosphate backbone
Each linear strand of nucleotides has a 5’ and and a 3’ end
The nitrogenous bases are perpendicular to the sugar phosphate backbone
Differences between DNA and RNA
DNA contains deoxyribose and RNA contains ribose
DNA contains thymine and RNA contains uracil
RNA is usually couble stranded ; RNA is usually single stranded
The two DNA strands in double stranded DNA are antiparallel
Key Takeaways
Both DNA and RNA are formed from nucleotide subunits connected by covalent bonds to form linear molecule with 5’ and 3’ ends. Each nucleotide is comprised of a sugar, phosphate group, and nitrogenous base
Differences include the type of sugar, one of the nitrogenous bases (RNA has uracil whereas DNA contains thymine), and number of strands. DNA has two nucleotide strands that are antiparallel
Unit 2
2.1 Cell Structure : Subcellular Components
Subcellular components universal to all cells
all living cells contain a genome and ribosomes, reflecting the common ancestry of all known life
Ribosomes synthesize protein according to mRNA sequences and the instructions that are encoded in that mRNA sequence originate from the genome of the cell
structure and function : ribosomes
ribosomes consist of two subunits that are not membrane enclosed
Ribosomes are made of ribosomal RNA and proteins
Ribosomes synthesize protein according to mRNA sequences
Structure and function : endoplasmic reticulum
the endoplasmic reticulum is a network of membrane tubes within the cytoplasm of eukaryotic cells
Two forms of ER
Rough ER
has ribosomes attatched to its membrane
compartmentalizes the cell
rough ER is associated with packaging the newly synthesized proteins made by attatched ribosomes for possible export from the cell
Smooth ER
does not have ribosomes attatched
functions include detoxification and lipid synthesis
Structural differences between rough ER and smooth ER leads to functional differences
Structure and function : golgi complex
series of flattened membrane bound sacs found in eukaryotic cells
involved in correct folding and chemical modification of newly synthesized proteins and packaging for protein trafficking
Structure and function : mitochondria
has a double membrane
outer membrane is smooth and inner membrane is highly convuluted, forming folds called cristae
Functions in production of ATP energy that eukaryotic cells can use for work
Structure and function : lysosomes
membrane enclosed sacs found in some eukaryotic cells that contain hydrolytic enzymes
hydrolytic enzymes can be used to digest a variety of materials such as damaged cell parts or macromolecules
Structure and function : vacuoles
membrane bound sacs found in eukaryotic cells
plays a variety of roles ranging from storage of water and other macromolecules to the release of water from a cell
Structure and function : chloroplasts
found in eukaryotic cells such, as photosynthetic agae and plants
double outer membrane
specialized for capturing energy from the sun and producing sugar for the organism
Key takeaways
ribosomes are not enclosed by a membrane and are subcellular components found in all forms of life, reflecting the common ancestry of all known life. Ribosomes function to synthesize proteins for cells.
Eukaryotic cells have additional membrane enclosed organelles that perform specialized functions for the cell. These include the rough er, smooth er, golgi complex, mitochondria, lysosomes, vacuoles, and chloroplasts
2.2 Cell structure and function
Structure and function : chlorplasts
specialized for photosynthesis and capturing energy from the sun to produce sugar
Within chloroplasts are distinct compartments
thylakoids :
highly folded membrane compartments that are organized in stacks called grana
Membranes contain chlorophyll pigments that comprise the photosystems and electron transport proteins can be found between the photosystems, embedded in the thylakoid membrane
Light dependent reactions occur here
Folding of these internal membranes increases the efficiency of these reactions
Stroma
fluid between the inner chloroplast membrane and outside thylakoids
the carbon fixation reactions occur here
Structure and function : mitochondria
double memrbane provides compartments for different metabolic reactions
Mitochondria capture energy from macromolecules
The krebs cycle reactions occur in the matrix of the mitochondria
Electron transport and ATP synthesis occur in the inner mitochondrial membrane
Folding of the inner membrane incereases the surface area, which allows for more ATP to be made
Structure and function : vacuoles
vacuoles play a variety of roles, including storage and release of water, macromolecules, and cellular waste products
in plants, vacuoles aid in retention of water for turgor pressure
turgor pressure is an internal cellular force, usually caused by water pushing up against the plasma membrane and cell wall
Structure and function : lysosomes
contain hydrolytic enzymes and can contribute to cell function in the following ways :
intracellular digestion
recycling of organic materials
programmed cell death (apoptosis)
Structure and function : endoplasmic reticulum
the ER performs the following functions for the cell
provides mechanical support
plays a role in intracellular transport
rough er carries out protein synthesis on ribosomes that are bound to its membrane
Key takeaways
Subcellular components and organelles interact to support cell function. The ER, mitochondria, lysosomes, and vacuoles, each have specialized functions that occur within their membrane enclosed structures which increases the efficiency of the cell to perform chemical reactions and store materials
Chloroplasts and mitochondria are structural features of eukaryotic cells that allow organisms to capture, store, and use energy. The folding og the inner membrane in both these structure increases the surface area which allows for more ATP to be synthesized.
2.3 Cell Size
Cells are typically small
moving materials in and out of cells gets more difficult the larger a cell is
Effects of surface area to volume ratios on the exchange of materials
smaller cells typically have a higher surface area to volume ratio and more efficient exchange of materials with the environment
As cells increase in volume, the relative surface area decreases making it difficult for larger cells to meet the demand for internal resources and remove waste sufficiently
these limitations can restrict cell size and shape
Key Takeaways
Smaller cells typically have a higher surface area to volume ratio and more efficient exchange of materials with the environment
Surface area to volume ratio affects the ability of a biological system to obtain necessary resources, eliminate waste products, acquire and dissipate thermal energy, and otherwise exchange chemicals and energy with the environment
Using the formulas given on the AP formula sheet, surface area and volume calculations can be done for a variety of cell shapes. Using calculated surface area and volume, a ratio can be determined
2.4 Plasma Membrane
Cells have membranes that allow them to establish an internal environment
cell membranes provide a boundary between the interior of the cell and the outside environment
Cell membranes control the transport of materials in and out of the cell
Phospholipids have both hydrophyllic and hydrophobic regions
phospholipids are amphipathic
hydrophyllic phosphate head is polar
Hydrophobuc fatty acid tail is nonpolar
Phospholipids spontaneously form a bi layer in aqueous environment
tails are located inside the bilayer
Heads are exposed to the aqueous outside and aqueous inside environments
Embedded proteins can be hydrophyllic or hydrophobic
peripheral proteins
loosely bound to the surface of the membrane
hydrophyllic with charged and polar side groups
Integral proteins
span the membrane
Hydrophilic with charged and polar side groups
Hydrophobic with nonpolar side groups penetrate hydrophobic interior of bilayer
Embedded proteins play various role sin maintaining the internal environment of the cell
membrane protein functions
transport
cell cell recognition
enzymatic activity
signal transduction
intercellular joining
attatchment for extracellular matrix or cytoskeleton
The framework of the cell membranes is described as the fluid mosaic model
structured as a mosaic of protein molecules in a fluid bilayer of phospholipids
The structure is not static and is held together primarily by hydrophobic interactions which are weaker than covalent bonds
Most lipids and some proteins can shift and flow along the surface of the membrane or across the bilayer
Fluid mosaic model includes steroids
chlosterol, a type of steroid, is randomly distributed and wedged between phospholipids in the cell membrane of eukaryotic cells
cholesterol regulates bilayer fluidity under different environmental conditions
Fluid mosaic model components include carbohydrates
diversity and location of the carbohydrates and lipids enable them to function as markers
glycoproteins : one or more carbohydrate attached to a membrane protein
Glycolipids : lipid with one or more carbohydrate attatched
Key Takeaways
Phospholipids spantaneously form a bi layer in an aqueous environment with hydrophilic phosphate regions oriented toward the aqueous external or internal envronment and the hydrophobic fatty acid regions face each other within the interior of the membrane
Embedded proteins can be hydrophilic, with charge and polar side groups, and or hydrophobic with nonpolar side groups
Embedded proteins have a variety of functions including transport, cell to cell regognition, enzym activity, signal transduction, intercellular joining, and attatchement to cytoskeleton and extracellular matrix
The fluid mosaic model consists of structural framework of phospholipid molecules that are embedded with proteins, steroidsm glycoproteins, and glycolipids that can flow around the surface of the cell within the membrane
2.5 Membrane permeability
The structures of the cell membrane
phospholipids are amphipathic
hydrophilic phpshate head is polar
hydrophobic fatty acid tail is nonpolar
Phospholipids spontaneously form a bi later in an aqueous environment
Fluid mosaic model : a moving phospholipid bilayer composed of varying types of molecules
Selective permeability is a direct consequence of membrane structure
The cell membrane is selectively permeable
small nonpolar molecules pass freely
N2
O2
CO2
Hydrophilic substances such as large polar molecles and ions can not freely move across the membrane
Hydrophilic substances move through transport proteins
channel proteins : a hydrophilic tunnel spanning the membrane the allow specific target molecules to pass through
Carrier proteins : spans the membrane and change shape to move a target molecules from one side of the membrane to the other
Small polar molecules can pass directly through the membrane in minimal amounts
The cell wall is a structural boundary and permeable barrier
as a structural boundary
protects and maintains the shape of the cell
prevents against cellular rupture when internal water pressure is high
Helps plants stand up against the force of gravity
As a permeable barrier
plasmodesmata : small holes between plant cells that allows the transfer of nutrients, waste, and ions
Animal cells do not have cell walls
Cell walls are composed of complex carbohydrates
cell wall : composed of complex carbohydrates
plants : cellulose
Polysaccharide
Fungi : chitin
polysaccharide
Prokaryotes : peptidoglycan
polymer consisting of sugar and amino acids
Key Takeaways
The structure of cell membranes results in selective permeability
Cell membranes separate the internal envornment of the cell from the external environment
Selective permeability is a direct consequence of membrane strucutre, as described by the fluid mosaic model
Small nonpolar molecules freely pass across the membrane
Hydrophilic substances move across the membrane through embedded channel and transport proteins
Polar uncharged molecules pass through the membrane in small amounts
Cell walls provide a structural boundary, as well as a permabilirt barrier for some substances to the internal environments
Cell walls of plants, prokaryotes, and fungi are composed of complex carbohydrates
2.6 Membrane transport
Selectively permeable membranes allow for the formation of concentration gradients
concentration gradient is when a solute is more concentrated in one area than another
A membrane separates two different concentrations of molecules
Passive transport is the net movement of molecules from a high to low concentration
net movement of molecules from high concentration to low concentration without metabolic energy such as ATP
Plays a primary role in the import of materials and the exports of wastes
Diffusion : movement of molecules from high concentration to low concentration s
small nonpolar molecules pass freely
Facilitated diffusion : movement of molecules from high concentration to low contration through transport proteins
allows for hydrophilic molecules and ions to pass through membranes
Active transport requires energy
active transport requires the direct input of energy to move molecules from regions of low concentrations to regions of high concentrations
Endocytosis requires energy to move large molecules into the cell
in endyocytosis, the cell uses energy to take in macromolecules and particulate matter by forming new vesicles derived from the plasma membrane
phagocytosis : cell takes in large particle
pinocytosis : cell takes in extracellular fluid containing dissolved substances
receptor mediated endocytosis : receptor proteins on the cell membrane are used to capture specific target molecules
Exocytosis requires energy to move large molecules out of the cell
in exocytosis : internal vesicles use energy to fuse with the plasma membrane and secrete large macromolecules out of the cell
proteins such as signaling proteins
hormones
waste
Key Takeaways
Passive transport is the net movement of molecules from high concentration to low concentration without the direct input of metabolic energy
Active transport requires the direct input of energy to move molecules from regions of low concentration to regions of high concentration
the selective permeability of membranes allows for the formation of centration gradients of solutes across the membrane
in exocytosis, internal vesicles use energy to fuse with the plasma membrane and secrete large macromolecules out of the cell
in endocytosis, the cell uses energy to take in macromolecules and particulate matter by forming new vesicles derived from the plasma membrane
2.7 Facilitated diffusion
Membrane proteins are necessary for facilitated diffusion
facilitated diffusion : movement of molecules from high concentration to low concentration through transport proteins
large and small polar molecules
largeg quantities of water can pass through aquaporins
charged ions require channel proteins
Active transport establishes and maintains concentration gradients
active transport moves molecules and ions against their concentration gradient from low to high concentration
carrier proteins called pumps require metabolic energy
establishes and maintains concentration gradients
Membrane proteins are necessary for active transport
cotransport : secondary active transport that uses the energy from an electrochemical gradient to transport two different ions across the membrane through a protein
symport : two different ions are transported in the same direction
Antiport : two different ions are transported in opposite directions
Membranes may become polarized by movement of ions
the cell membrane allows for the formation of gradients
electrochemical gradients
type of concentration gradient
membrane potential : electrical potential difference across a membrane
membranes may become polarized by movement of ions across a membrane
The NA+ K+ ATPase contributes to membrane potential
NA+ K+ ATP ase contributed to the maintenance of the membrane potential
3 NA+ pumped
2 K+ pumped
Key takeaways
membrane proteins are required for facilitated diffusion of charged and large polar molecules through a membrane
large quantities of water pass through aquaporins
Membranes become plarized by the movement of ions across the membrane
Membrane proteins are necessary for active transport
Metabolic energy is required for active transport of molecules and ions across the membrane and to establish and maintain concentration gradients
The NA+ K+ ATPase contributes to the maintenance of the membrane potential
2.8 Tonicity and Osmoregulation
Water moves by osmosis
osmosis is the diffusion of free water across a selectively permeable membrane
large quantities of water move via aquaporins
Osmolarity is the total solute concentration in a solution
water has high solvency abilities
solute is the substance being dissolved
solvent is a substance that dissolves a solute
Solution is a uniformed mixture of one ore more solutes dissolved in a solvent
Tonicity effects a cells physiology
tonicity is the measurement of the relative concentrations of solute between two solutions
Internal cellular environments can be hypotonic, hypertonic, or isotonic to external environments
hypotonic
more solute less solvent
Hypertonic
less solute more solvent
isotonic
equal concentrations of solute and solvent
Water moves by osmosis to the area with a higher solute concentration
water concentrations and solute concentrations are inverselt related
water would diffuse out of a hypotonic environment to a hypertonic environment
solutes diffuse along their own concentration gradients, from the hypertonic environment into the hypotonic environment
When a cell is in an isotonic environment, a dynamic equilibrium exists with equal amounts of water moving in and out of the cell at equal rates
no net movement of water takes place
Osmoregulator mechanisms contribute to survival
in plant cells, osmoregulation maintains water balance and allows control of interal solute composition / water potential
Environmental hypertonicity
less cellular solute and more cellular water
plasmolysis
Isotonic solution
equal solute and water
flaccid
Environmental hypotonicity
more cellular solute and less cellular water
turgid
The cell wall helps maintain homeostasis for the plant in environmental hypotonicity
osmotic pressure is high outside of the plant cell due to environmental hypotonicity
water flows into the plant vacuoles via osmosis causing the vacuoles to expand and press against the cell wall
The cell wall expands until it begins to exert pressure back on the cell, this pressure is called tugor pressure
turgidity is the optimum state for plant cells
In animal cells, osmoregulation maintains water balance and allows control of internal solute composition / water potential
environmental hypertonicity
less cellular solute and more cellular water
shriveled
isotonic solution
equal solute and water
normal
environment hypotonicity
more cellular solute and less cellular water
lysed
Key Takeaways
external environments can be hypotonic, hypertonic, or isotonic to the interal envornment of cells
Water moves by osmosis from areas of low osmolarity/solute concentraation to area of high solute concentration
Growth and homeostasis are maintained by the constant movement of molecules across membranes
Osmoregulation maintains water balance and allows organisms to control their internal solute composition
The components of an effective graph
title
experiment details and what is being measured
Labeled axes with units
Line graph
reveals trends or progress over time for multiple groups or treatments
track chanegs over time concentrations
XY graph
scatterplot
to determine relationships between two different things
compare two variables that may or may not have a linear relationship
Histogram
show how values in a data set are distributed across evenly spaced or equal intervals
explore the relationship between two or more variables
Bar graph
compare multiple groups or treatments to each other
Box and whisker plots
show the variability in a sample
ideal for comparing distributions in relation to the mean
Key takeaways
the components of a graph include a title, correctly labeled axis with units, unifromed intervals, identifiable lines or bars, and trend lines
The graph type used is based on the type of data collected
Graphs can be used to show trends over time, comparisons, distributions, correlations variability in samples, and relationships between variables
Water moves by osmosis
water potential measures the tendency of water to move by osomsis
calculated from pressure potential and solute potential

Water moves from an area of high water potential to an area of low water potential
The more negative the water potential, the more likely water will move to the area
Osmoregulation allows organisms to control their internal solute composition and water potential
increasing the amount of solute in water will cause
increase solute potential
decreased water potential
Increasing water potential
an increase in pressure potential
Decreasing pressure potential will cause
decrease in water potential
Water potential is equal to solute potential

Key Takeaways
Water moves by osmosis from areas of high water potential to areas of low water potential
Water moves by osmosis from areas of low solute potential to areas of high solute potential
Osmoregulation maintains water balance and allows organisms to control their internal solute composition/water potential
2.9 Mechanisms of Transport
Passive transport is the net movement of molecules down their concentration gradient
diffsuion : movement of molecules from high concentration to low concentraiton
small nonpolar molecules pass freely across a cell membrane
small amounts of very small polar molecules can diffuse across a cell membrane
facilitated diffusion : movement of molecules from high concentration to low concentration through transport proteins
large and polar molecules
charged ions require channel proteins
Osmosis is the diffusion of water across a selectively permeable membrane
large quantities of water move via aquaporins
Differences in relative solute concentrations can facilitate osomosis
Active transport is the movement of molecules against their concentration gradient
active transport moves molecules and or ions against their concentration graident, from low concentration to high concentration
protein pumps are carrier proteins used in active transport
requires metabolic activity
establishes and maintains concentration gradients
Movement of large molecules into and out of cells requires energy
in endocytosis, the ccell uses energy to take in macromolecules and particulate matter by forming new vesicles derived from the plasma membrane
the types of endocytosis are phagocytosis, pinocytosis, and receptormediated endocytosis
In exocytosis, internal vesicles use energy to fuse ewith the plasma membrane and secrete large macromolecules out of the cell
Key Takeaways
Passive transport is the net movement of molecules from high concentration to low concentration without direct inpiut of energy
Water is transported in small amounts across the membrane by simple diffusion and in large amounts via facilitated diffusion through aquaporins embedded in the membrane
Active transport requires the direct input of energy to move molecules from regions of low concentration to regions of high concentration
Large molecules and large amounts of molecules are moved into the cell by endocytosis and out of the cell by exocytosis
2.10 Compartmentalization
Compartmentalization in Eukaryotic cells
cells have a plasma membrane that allow them to establish and maintain internal environments that are different from their external environments
Eukaryotic cells have additional internal membranes and membrane bound organelles that compartmentalize the cell
Cellular compartments allow for various metabolic processes and specific enzymatic reactions to occur simultaneously, increasing the efficiency of the cell
Cellular compartments : lysosomes
membrane minimizes competing interactions
The hydrolytic enzymes of the lysosome function at an acidic environment
By having this compartmentalization, th inside of the lysosome can maintain a more acific pH and allow for efficient hydrolysis to occur
cellular compartments : mitochondria
membrane folding maximizes surface area for metabolic reactions to occur
electron transport and ATP synthesis occur in the inner mitochondrial membrane
folding of the inner membrane increases the surface area, which allows for more ATP to be made
Cellular compartments : chloroplasts
membrane folding maximizes surface area for metabolic reactions to occur
the thylakoids are highly folded membrane compartments that increase the efficienty of the light dependent reactions
Key takeaways
eukaryotic cells contain various membrane bound organelles.These structures compartmentalize intracellular processes and enzymatic reactions increasing the efficiency of cellular function
Internal membranes facilitate cellular processes by minimizing competing interactions and by increasing surface areas where reactions can occur
Loss of these intraceullular compartments or changes to unique internal surfaces and environments within membrane bound organelles may hinder proper cell function
2.11 Origins of cell compartmentalization
Comparison of compartmentalization in prokaryotic and eukaryotic cells
both cell types have plasma membrane that separates their internal environment from their surrounding environment
Prokaryotic cells have an internal region, nucleoid region, that contains its genetic material
Eukaryotic cells have additional internal membranes and membrane bound organelles that compartmentalize the cell
genetic material is contained within a membrane bound nucleus
The evolution of membrane bound organelles
the nucleus and other internal membranes are theorized to have formed from the infoldings of the plasma membrane
Mitochondria evolved from previously free living prokaryotic cells via endosymbiosis
a free living aerobic prokaryote was engulfed by an anaerobic cell through endocytosis
the engulfed cell became beneficial to the cell
Key takeaways
Both prokaryotic and eukaryotic ccells have external plasma membranes. However, whereas prokaryotes only have internal regions where specialized structures and function can occur, eularyotic cells have additional internal membrane bound organelles that compartmentalize the cells
According to the theory of embosymbiosis, a previously free living prokaryotes was engulfed by another cell through endocytosis
Evidence supporting the evolution of mitochondria and chlorplasts via endosymbiosis includes the presence of double membranes, circular dna, and ribosomes in both these organelles
Unit 3
3.1 Enzyme Structure
what are enzymes
enzymes are macromolecules
biological catalysts that speed up biochemical reactions
most enzymes are proteins
have a tertiary shape that must be maintained
what is an active site?
interacts with the substrate (a molecule that interacts with the active site of an enzyme)
enzymes have an active site specific to the substrate (unique shape and size + can have charges + must have specific properties that are compatible with the substrate)
enzyme names often indicate the chemical reactions involved
often end in -ase (sucrase: digests sucrose)
Enzymes are reusable
they are not chemically changed by the reaction
cells usually maintain a specific enzyme concentration
Enzymes can facilitate synthesis or digestion reactions
Enzymes speed up reactions by lowering activation energy requirements
The structural characteristics of an enzyme make the reactions very enzyme-specific
the shape and charge of the substrate must be compatible with the active site of the enzyme for a reaction to occur
enzymes are not consumed by a reaction; they are reused
3.2 Enzyme Catalysis
each enzyme only facilitates one specific reaction (enzymes are really specific)
what is activation energy?
the initial starting energy
some reactions result in a net absorption of energy and others result in a net release of energy
typically reactions resulting in a net release of energy require less activation energy compared to reactions that absorb energy
What do enzymes do? : they lower activation energy, which accelerates the reactions
A controlled experiment is a scientific investigation
there are 2 groups
control : they’re intentionally not changed
generates data under conditions with no manipulation
generates data under normal conditions
considered baseline data
negative control : not exposed to manipulation or any treatment
positive control : exposed to a treatment that has a known effect
not exposed to experimental effect
experimental group
generates data under abnormal/unknown conditions
generates data under manipulated conditions
often compared with the control group to determine impacts of manipulation
3.3 Environmental Impacts on Enzyme Function
Enzymes may have unique conformational shapes : different tertiary structures
changes in these shapes is denaturation
denaturation can occur to :
changes in temperature
changes in environmental pH
denaturation is typically irreversible (catalytic ability of the enzyme is lost or significantly decreased)
in some cases, its reversible
Enzymes have optimum temperatures
range in which their mediated reactions occur the most efficiently
reaction rates change when the optimum temperatures arent maintained
When there is an environmental increase in temperature
the reaction rate typically increases: increased speed of molecular movement
temperature increases outside of the optimum temperatures, result in denaturation
When there is an environmental decrease in temperature
the reaction rate is generally slowed
decrease the frequency of enzyme-substrate collisions
does not interrupt enzyme structure ; no denaturation
pH changes can affect enzyme activity
pH measures the amount of hydrogen ions in a solution
Optimum pH
same thing as optimum temperature : range in which enzyme mediated reactions occur the fastest
changing the pH out of the range will slow or stop enzyme activity
enzyme denaturation can occur
When the substrate concentration increases
initially, the reaction rate increases
more substrates means more of a chance to collide with the enzyme
substrate saturation will eventually occur, there will be no further increase in the rate —> the reaction rate will remain constant if the saturation levels remain the same
changes in enzyme concentration can also change reaction rates
less enzymes = slower reaction rate
less opportunity for substrates to collide with an active site
more enzymes = higher reaction rate
more opportunity for substrates to collide with an active site
Competetive inhibitor : molecules can bind reversibly or irreversibly to the active site of an ezyme
competes with normal substrate for the enzymes active site
if inhibitor reactions exceed substrate : the reactions are slowed
if inhibitor binding is irreversible, enzyme activity will be prevented and vis versa.
Enzymes can have regions other than the active sites called the allosteric sites
noncompetitive inhibitors : do not bind to the active site
causes conformational shape change
binding prevents enzyme function because the active site is no longer available → reaction rate decreases
3.4 Cellular Energy
All living systems require a constant input of energy
sunlight is the main source of energy
autotrophs capture sunlight and turn it into useable energy by all sources
during some energy transformations, energy is typically lost (as heat)
Every energy transfer increases the disorder of the universe
living cells are not at equilibrium: there is constant flow in and out of the cell
Cells maintain energy by energy coupling
energy releasing processes drive energy-storing processes
Within cells, the product of one reaction can serve as the reactant for another
3.5 Photosynthesis
Structure of Chloroplast
surrounded by double membrane
central fluid filled space : stroma
system of interconnected membranous sacs : thylakoids
stacks of thylakoids : grana
fluid filled compartment in thylakoids : lumen
Two stages of photosynthesis
Light reactions (light dependent reactions)
convert solar energy to the chemical energy of ATP and NADPH
happens in thylakoid
noncyclic electron flow
linear flow of electrons
move in one direction from water to NADPH
create a concentration gradient of H+ that drives the production of ATP through ATP sythase
Cyclic electron flow
only produces ATP (no NADPH no OXYGEN)
makes more ATP
calvin cycle (light independent reactions)
uses energy from the light reactions to incorporate CO2 from the atmosphere into sugar
happens in stroma
Carbon fixation : carbon dioxide is attatched to RuBP
carbon is unstable and immediately splits in to 2 3-PGA
Reduction : ATP provides energy / NADPH provides power to reduce intermediates
6 G3P are produced —> 1 leaves the cycle leaves the cycle to be made in glucose
Regeneration : rest of the G3P rearrange to regenerate the starting RuBP molecules
3.6 Cellular Respiration
OILRIG : oxidation and reduction are coupling reactions
Photosynthesis and cellular respiration are coupling reactions.
oxidation: losing electrons
reduction : gaining electrons
Cellular respiration
the process by which cells convert glucose and oxygen into energy, carbon dioxide, and water. This process can be divided into three main stages: Glycolysis, the Krebs cycle, and the Electron Transport Chain.
Cellular respiration is the catabolism of organic molecules within cells to generate energy, ATP.
equation : C6H12O6 + 6O2 —> 6CO2 + 6H2O + energy (ATP)
1) Glycolysis
happens in the cytoplasm
Glucose cannot fit into the mitochondrial matrix naturally, so it must be broken down.
Six-carbon glucose is converted into two 3-carbon molecules of pyruvate.
Step 1 -3 : 2 ATP are input and broken down into ADP (endergonic reactions —> require energy) ; the bonds between the 2nd and 3rd phosphates are high energy bonds, so the energy released from these bonds in the early steps of glycolysis powers the rest of the reaction
Step 4 : the six-carbon sugar is split into two 3-carbon sugars
step 5 -10 : the three carbon sugars are further processed through exergonic reactions, producing 4 ATP molecules and 2 NADH molecules
the net gain for glycolysis of one glucose molecule is two molecules of ATP and 2 NADH
NADH and NAD+ are intermediary molecules that transport electrons
2) Pyruvate Oxidation
Pyruvate oxidation occurs in the mitochondrial matrix.
Pyruvate oxidation links glycolysis and the citric acid cycle by oxidizing (taking away electrons) from pyruvate to form acetyl coA
Step 1 : pyruvate undergoes decarboxylation —> the two 3-carbon sugars lose 2 CO2
—> NAD+ is released
—> NAD+ is reduced (gains electrons) to form NADH
—> One molecule of NADH is produced per pyruvate, resulting in 2 NADH molecules
—> NADH is reduced to capture the high-energy electrons that are found in NAD+
2 Acetyl CoA is produced: the main purpose is for its acetyl group to be donated to the four-carbon compound oxaloacetate to form the six-carbon molecule citrate
3) Krebs Cycle (Citric Acid Cycle)
Acetyl CoA is the starting point for the citric acid cycle; the pathway of 8 reactions oxidizes the two-carbon acetyl group to two molecules of CO2
Step 1: Acetyl CoA
Step 2 : oxidized: it loses 2 electrons, which are given to the 4 carbon molecule oxaloacetate to form citrate
Step 2-3: NAD+ is reduced to form NADH, loses a CO2 (this happens 2 times so 2 carbons are lost —> forms a four-carbon molecule)
Step 8: NAD+ is reduced again to regenerate oxaloacetate
the citric acid cycle harvests energy from the oxidation of acetyl CoA
4) Electron transport chain
a series of redox carrier proteins embedded in the inner membrane of the mitochondrion
the electron transport chain takes NADH and oxidizes it to NAD+, so the electrons and hydrogens pass through the carrier proteins to the outer mitochondrial matrix, thus setting up a concentration gradient
the H+ ions move through the final carrier protein, ATP synthase which uses the H+ gradient to synthesize ATP by chemiosmosis
chemiosmosis is when the ATP synthase receives the H+ ions, a spinning enzyme begins to rotate which causes ADP to gain phosphate and become ATP
the H+ ions are passed back to the inner mitochondrial matrix, where they are attracted to oxygen —> the oxygen is reduced to H2O to keep the concentration gradient higher on the outside so H+ keeps going through the ATP synthase
FOR EVERY GLUCOSE MOLECULE 34-38 ATP ARE CREATED UNDER AEROBIC CONDITIONS
Fermentation
under anaerobic conditions, the electron transport chain cannot operate and NADH produced by glycolysis would not be reoxidized, causing glycolysis to stop because there would be no NAD+ for step 6 of glycolysis —> to solve this problem organisms use fermentation to reoxidize the NADH
Fermentation pathways occur in the cytoplasm
only 2 ATP per glucose is made in fermentation (restricted to the amount of glucose made in glycolysis because fermentation doesn’t go further than the cytoplasm and glycolysis)
Lactic acid fermentation
glycolysis occurs
pyruvate serves as the electron acceptor and the product is lactate
NADH oxidized back to NAD+
reversible
Alcoholic fermentation
glycolysis occurs
pyruvate is converted to ethanol
NADH oxidized back to NAD+ & CO2 is a byproduct
reversible
3.7 Fitness
cells can vary
molecular shape
molecular types (carbohydrates, lipids)
variation increases fitness
Individual fitness
refers to an individual organisms ability to survive and reproduce
individual fitness connects to species fitness
the more variation a species has, the more likely the species is to thrive and survive
Unit 4
4.1: Cellular Communication
How do cells communicate with one another
Cells can signal to themselves (autocrine)
direct contact with other cells (juxtacrine)
cells of multicellular organisms maintian physical contact with other cells
cells can also send chemical signals into adjacent cells (paracrine)
cell membrane and cell wall modifications aid in this (ex. plant cells have plasmodesma + in animal cells, there are gap junctions)
Cells communicate over short and long distances
the cell recieving the signal is referred to as the target cell
short distance
cell sends out local regulator (local = close)
target cell is within short distance of the signal (local signalling)
often used to communicate with cells of the same type
same communication
close proximity
long distance (endocrine)
target cell is not in the same area as the signal
signal travels a long distance
often used to signal cells of other type
Key takeaways
cells use local regulators to communicate across short distances
cells can release chemical signals with the ability to travel over long distances to target cells of other types
structural modifications of the cell membrane and cell wall allows cells to send signals directly into adjacent cells
4.2: Introduction to Signal Transduction
signal transduction pathways link signal reception with cellular responses
3 steps of cell communication
reception
detection of a signal molecule coming from outside the cell
transduction
how the signal is converted to a form that can bring about a cell response
response
specific cellular response to the signal molecule
many signal transduction pathways include protein modification and phosphorylation cascades
regulation protein synthesis by turning genes on or off in the nucleus
regulate activity of proteins in the cytoplasm
cascades of molecular interactions relay signals from receptors to target molecules
Phosphorylation cascade : enhance and amplify signal
Signalling begins with the recognition of a chemical messenger, a ligand, by a receptor protein in a target cell
the ligand binding domain of a receptor recognizes a specific chemical messenger in a specific one to one relationship
Signalling cascades relay signals from receptors to cell targets, often amplifying the incoming signals, resulting in the appropriate responses by the cell
after the ligand binds, the intracellular domain of a receptor protein changes shape, initiating transduction of the signal
secondary messangers are molecules that relay and amplify the intracellular signal
binding of ligand to ligand gated channels can cause the channel to open or close
Large, polar molecules typically bind to extracellular receptors while small, nonpolar molecules bind to intracellular receptors
Key takeaways :
A singal transduction pathway is the binding of singalling molecules to receptors located on the cell surface or inside the cell that trigger events inside the cell, to invole a response
Cells use signal transduction pathways to link signal reception with cellular response
a singal transduction pathway begins when a receptor / ligand binds to external receptors or an intracellular receptor
The role of protein modification in signal transduction pathways is to cause a conformational shape change due to ligand binding. This change elicits an intracellular response, which causes a secondary messenger to be activated.
A phosphorylation cascade is a signalling pathway where one enzyme phosphorylates another, causing amplification of the reaction, leading to the phosphorylation of thousands of proteins
4.3 : Signal Transduction
Signal transduction pathways typically occur in 4 steps
signaling
reception
transduction
response
signaling and reception are typically one step
signal transduction pathways influence how the cell responds to its environment
the environment is not static, and organisms need to regulate pathways to respond to changes in the environment
the ability to respond to stimulus is a characteristic of life and necessary for survival
Signal transduction may result in changes in gene expression and cell function
singalling pathways can target gene expression and alter the amount or type of a particular protein produced in a cell
changes in protein production can result in phenotype changes
apoptosis can be a result of signal transduction
G Protein coupled receptors
look for the signal “moving over”
ligand binds to receptor and receptors shape changes
the receptor binds to the G protein
GDP is replaced with GTP to activate the G protein
the G protein binds to an enzyme to activate it —> leads to transduction and cellular response
Steroid Signalling
Happens inside the cell

Tyrosine Kinase receptor
“two to one”
dimer is formed
the bottoms of the dimer are usally connected with phosphate groups
Key Takeaways
The environment is not static and organisms need to respond to changes in the environment. The ability to respond to stimuli is characterstic of life and necessary for survival
Signal transduction pathways are used to influence cellular responses when the environment changes. Transduction pathways can regulate gene expression in response to changes in the environment or lead to apoptosis.
4.4: Changes in signal transduction pathways
Changes in signal transduction pathways can alter cell response
mutations in any domain of the receptor protein or in any component of the signaling pathway may affect the downstream components by altering subsequent transduction of the singal
change in protein structure can result in change of function
one disruption in the pathway can affect the downstream reactions
Chemicals that interfere with any component of the signalling pathway may activate or inhibit the pathway
Key Takeaways
A mutation that alters the ligand / receptor specificity can lead to incompatibility which can alter the signal transduction pathway. The receptor will not undergo proper conformational shape change, resulting in an inactive internal pathway.
Chemicals can activate a singal transduction pathway, which can lead to amplification of the pathway
Chemicals can inhibit a singal transduction pathway, which can lead to the pathway not occurring
4.5: Feedback
Organisms use feedback mechanisms to maintain their internal environments and respond to environmental changes
the internal and external cell environments are constantly changing
homeostasis is the maintenance of a stable internal environment
feedback mechanisms are processes used to maintain homeostasis by increasing or decreasing a cellular response to an event
Negative feedback mechanisms maintain homeostasis for a particular cell condition
Negative feedback mechanisms maintain homeostasis for a particular condition by regulating physiological processes
if a system is disrupted, negative feedback mechanisms return the systems back to its target set point
ex.
Human increases physical activity causes increased dissolved Co2 in the blood
The Co2 reacts with water in the blood to form a weak acid which lowers the pH of the blood
The medulla of the brain registers the drop in pH and signals to the diaphragm and heart to increase respiration
Co2 is cleared from bloodstream
Positive feedback mechanisms amplify responses and processes in biological organisms
the variable initiating the response is moved farther away from the initial set point, disrupting homeostasis
amplification occurs when the stimulus is further activated which initiates an additional response that produces system change
ex.
hypothalmus (brain) releases oxytocin
uterine walls contract
baby pushes against cervix
oxytocin continues to get released so long as baby is pushing against cervix (does not stop until baby is born)
Key Takeaways
feedback mechanisms are processes used to maintain homeostasis by increasing or decreasing cellular response to an event
negative feedback mechanisms maintain homeostasis for a particular condition by regulating physiological processes
positive feedback mechanisms amplify responses and process by regulating physiological processes
4.6: Cell cycle
The cell cycle is a highly regulated series of events for the growth and reproduction of cells
the cell cycle consists of two highly regulated processes
interphase
growth and preparation
M phase
mitosis : division of the nucleus
cytokinesis : division of the cytoplasm
Interphase involves 3 sesquential stages
G1 : cell growth
S : copies of DNA are made
G2 : the cytoplasmic components are doubled in preparation for division
In interphase, the cell undergoes growth processes and prepares for cell division.
In eukaryotes, cells transfer genetic infromation via highly regulated processes
mitosis plays a role in growth, tissue repair, and asexual reproduction
Mitosis ensures the transfer of a complete genome from a parent cell to two genetically identical daughter cells
Mitosis alternates with interphase in the cell cycle
Mitosis occurs in a sesquential series of steps (prophase, metaphase, anaphase, telophase)
Mitosis is followed by cytokinesis
cytokinesis ensures equal distribution of cytoplasm to both daughter cells
Cells can enter G0 phase where cell division no longer occurs, a cell can reenter the cycle with appropriate signals
Nondividing cells can exit the cell cycle or be held in a particular stage
Key Takeaways
the role of interphase is to allow newly divided cells the opportunity to grow, maintain normal cell function, and prepare for division
During interphase, cells grow, replicate DNA, and prepare for division
Mitosis plays a role in growth, tissue repaire, and asexual reproduction
During mitosis genetic information is transferred
Cytokinesis ensures equal distribution of cytoplasm to daughter cells
Mitosis is a process that ensures the transfer of a complete genome to both daughter cells
daughter cells carry genomes exactly identical to the parent cell genome
Prophase
nuclear envelope begins to disappear
DNA coils into visible chromosomes
fibers begin to move double chromosomes to the center of the cell
Separates the duplicated DNA and proteins contained in the nucleus

Metaphase
fibers align double chromosomes across
The nucleus dissolves and the cells chromsomes condense / align at center of cell
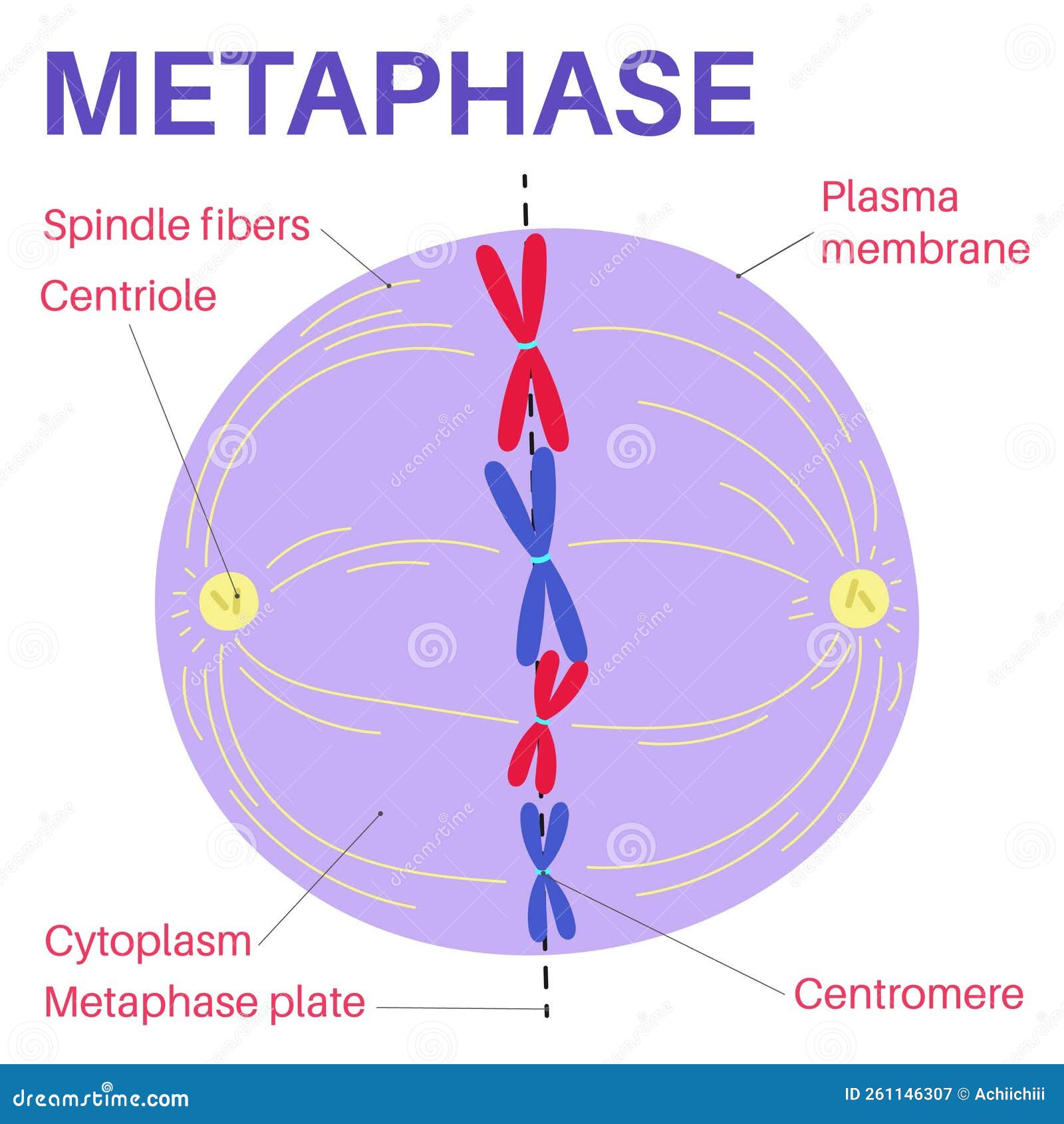
Anaphase
fibers separate double chromosomes into single chromosomes (chromatids)
chromosomes separate at the centromere
chromatids migrate to opposite sides of the cell
sister chromatids separate from eachother at centromere and pull to opposite sides of the cell by the spindle fibers (ensures that each daughter cell recieves a full set of chromosomes)
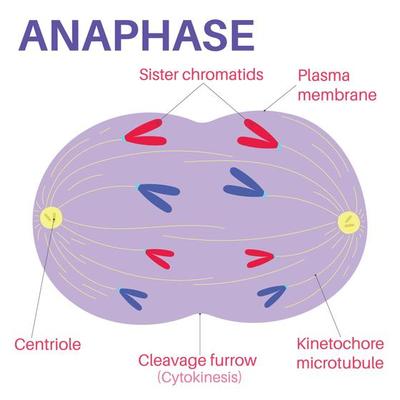
Telophase
nuclear envelope reappears and establishes two separate nuclei
each nucleus contains a complete genome
chromosomes will begin to uncoil
the nuclear membrane reforms around the DNA and he spindle fibers disassemble
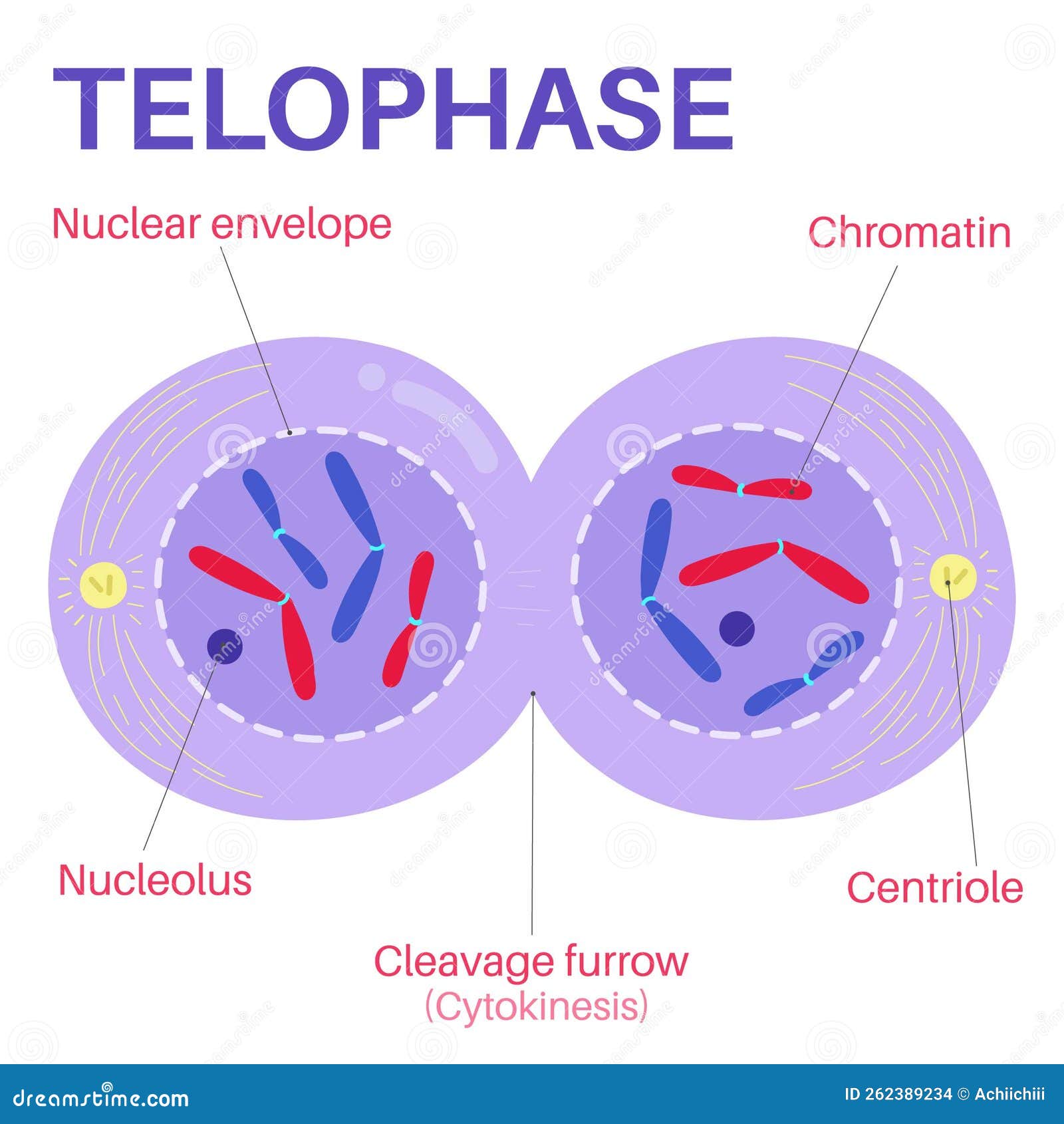
Cytokinesis will separate the cell into two daughter cells, each with identical genomes
each daughter cell gets equal cytoplasm

Key Takeaways
DNA becomes visible and nuclear envelope disappears during prophase
Chromosomes are aligned across center of the cell
Double chromosomes are separated into single chromsomes and single chromosomes align at opposite sides of the cell
DNA uncoils and nuclear envelope reappears. Two new nuclei form and each nucleus contains a complete genome
Cytokinesis begins at the end of mitosis and separates the cell into two daugter cells
4.7: Regulation of cell cycle
Internal controls or checkpoints regulate progression through the cycle
G1 Checkpoint
at the end of G1 phase
cell size check
nutrient check
growth factor check
DNA damage check
G2 checkpoint
at the end of G2
DNA replication check
DNA damage check
M spindle checkpoint
fiber attatchment to chromosome check
Interactions between cyclins and cyclin deprendent kinases control the cell cycle
Cyclins
a group of related proteins associated with specific phases of the cell cycle
different cyclins are involved in different phases of the cell cycle
Concentrations can fluctuate depending on cell activity
produced to promote cell cycle progression
degraded to inhibit cell cycle progression
Used to activate cyclin dependent kinases
cyclins are specific to the CDK they activate
Cyclin dependent kinases
group of enzymes involved in cell cycle regulation
requires cyclin binding for activation
phosphorylate substrates, promotes certain cell cycle activities
Disruptions to the cell cycle can result in cancer and/pr apoptosis
cell goes through cell cycle repeatedly without going through checkpoints
Key takeaways
checkpoints are regulatory events in the cell cycle
checkpoints help determine whether the cell is ready to progress through the cell cycle
proteins are used to activate or inhibit cell cycle activites
apoptosis and/or cancer can occur when the cell cycle is disrupted