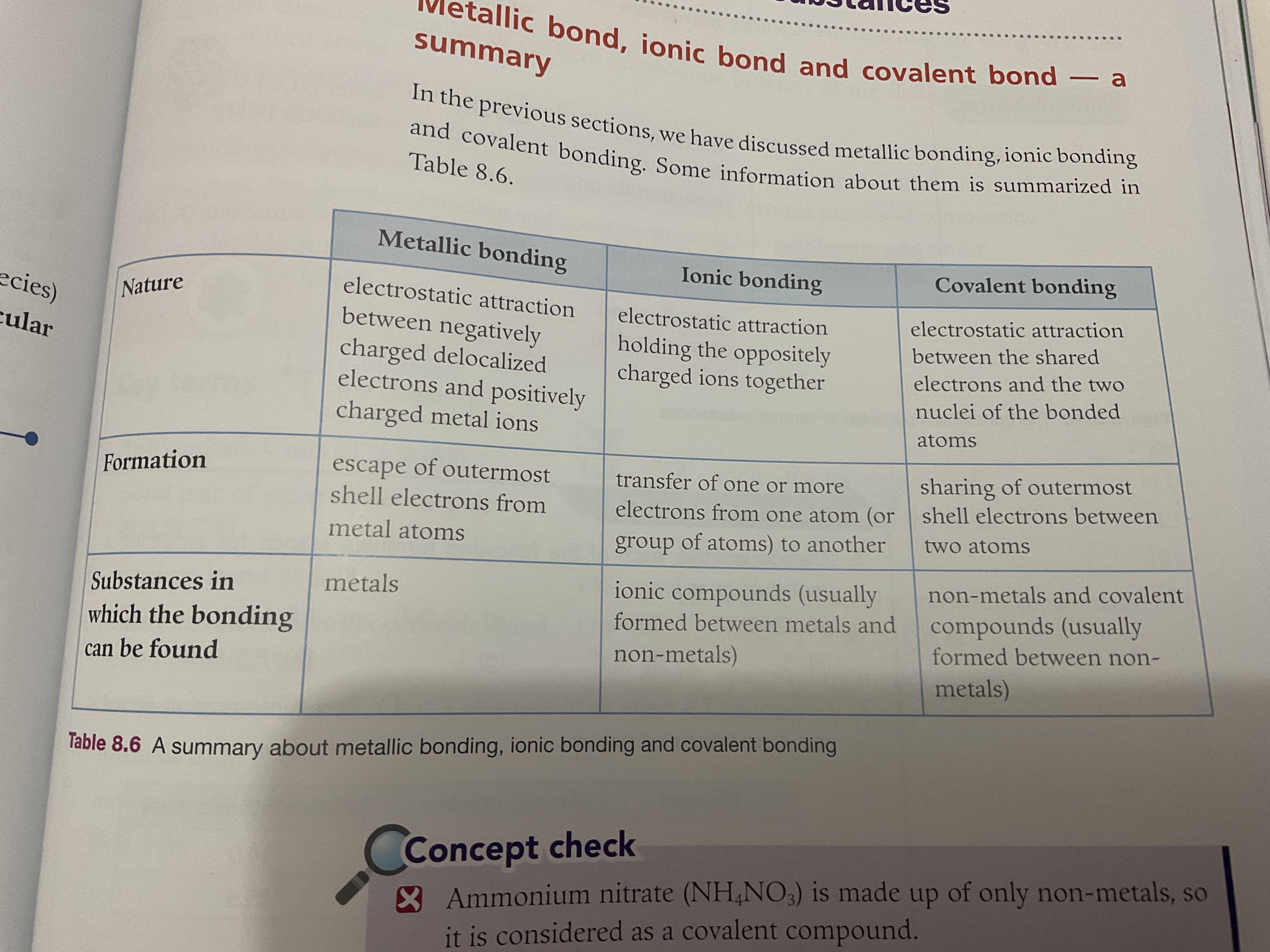
Covalent bond — The sharing of outermost shell electrons between two atoms. (atoms form molecules)
Nature: electrostatic reaction between shared electrons and two nuclei
Electrostatic attraction between shared electrons and two nuclei of the bonded atoms.
—Between non metals and non metals.
Diatomic molecules —molecules composed of two atoms, typically of the same element, such as O2 or N2.
Molecular formula = Cl2 (number of atoms) Structural formula = Cl — Cl (bond)
Single bond: —
Double bond: =
Triple bond: 三
Stable duplet = 2 electrons / Stable octet = 8 electrons
Examples:
Common Molecules: Look for common diatomic molecules that form triple bonds, such as nitrogen (N2), which has a triple bond between the two nitrogen atoms.
Valence Electrons: Check the valence electron counts: atoms involved in triple bonds typically require three pairs of electrons to achieve a stable octet.
names of compounds
Mono = 1
Di = 2
Tri = 3
Tetra = 4
To find the relative molecular mass of a compound, follow these steps:
Formula: Get the compound's formula (like H2O).
Atomic Mass: Check the periodic table for each element's mass (H = 1.01, O = 16.00.)
Calculate: Multiply each element's mass by how many atoms are in the formula.
Example: For H2O:
H: 1.01 × 2 = 2.02
O: 16.00 × 1 = 16.00
Add: Now, add those numbers together.
Formula mass (for all substances) = Relative molecular mass (only applies to molecular substances)
Dative covalent bond
A dative bond is a covalent bond formed by two atoms where both electrons in a shared pair are contributed from the same atom.
 Note
Note Studied by 11 people
Studied by 11 people Note
Note Studied by 52 people
Studied by 52 people Note
Note Studied by 48 people
Studied by 48 people Note
Note Studied by 7 people
Studied by 7 people Note
Note Studied by 10 people
Studied by 10 people Note
Note Studied by 153 people
Studied by 153 people Knowt
Knowt