Chapter 11 - Semen
Physiology
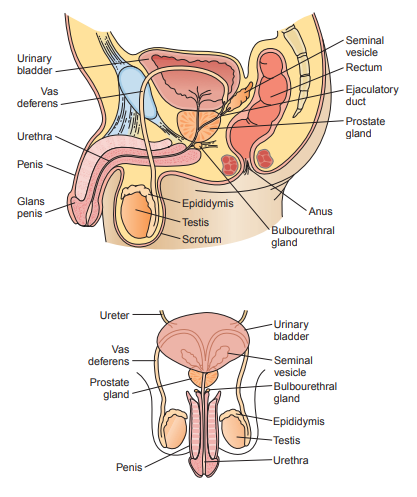
Seminiferous Tubules
- Sertoli cells provide support and nutrients for the germ cells as they undergo spermatogenisis, or the process of mitosis and meiosis in male gametes.
Epididymis
- After spermatogenisis, the sperm are still non-motile or unable to move, so they mature here and develop flagella.
- They remain stored in the epididymis until ejaculation, where they are propelled through the ductus deferens (vas deferens) to the ejaculatory ducts.
Seminal Vesicles
- The seminal vesicles produce the majority of the fluid present in semen (60% to 70%).
- The fluid contains a high concentration of fructose which provides energy for the sperm to move through the female reproductive tract.
Prostate Gland
- It provides 20% to 30% of the semen volume.
- It is acidic fluid containing high concentrations of acid phosphatase, citric acid, zinc, and proteolytic enzymes responsible for both the coagulation and liquefaction of the semen following ejaculation.
Bulbourethral Glands
They provide only 5% of the semen volume.
It is a thick, alkaline mucus that helps to neutralize acidity from the prostate secretions and the vagina’s bacteria flora.
Specimen Collection
- The majority of sperm are contained in the first portion of the ejaculate.
- Specimens are collected following a period of sexual abstinence of from 2 to 3 days to not longer than 5 days.
- Specimens collected following prolonged abstinence tend to have higher volumes and decreased motility.
- When performing fertility testing, two or three samples are usually tested at 2-week intervals, with two abnormal samples considered significant.
- The laboratory should provide warm sterile glass or plastic containers and a private room; however, if this is not appropriate, the specimen should be kept at room temperature and delivered to the laboratory within 1 hour of collection.
- Specimens awaiting analysis should be kept at 37°C.
- Specimens should be collected by masturbation or non-lubricant-containing rubber or polyurethane condoms, as ordinary condoms may have spermicidal properties.
Semen Analysis
Appearance
| Color | Clinical Significance |
|---|---|
| gray-white, translucent | normal |
| white turbidity | WBCs, infection within the reproductive tract |
| red coloration | RBCs |
| yellow | urine contamination, prolonged abstinence, medications |
Liquefaction
- A fresh semen specimen is clotted and should liquefy within 30 to 60 minutes after collection.
- If after 2 hours the specimen has not liquified, proteolytic enzymes such as alpha-chymotrypsin may be added to be able to continue performing tests.
- Failure of liquefaction to occur may be caused by a deficiency in prostatic enzymes and should be reported.
Volume
- It is measured by pouring the specimen into a clean graduated cylinder calibrated in 0.1-mL increments.
- Normal semen volume ranges between 2 and 5 mL.
- Increased volume may be seen following periods of extended abstinence.
- Decreased volume is more frequently associated with infertility and may indicate improper functioning of one of the semen-producing organs, primarily the seminal vesicles, or incomplete specimen collection
Viscosity
- The normal semen specimen should be easily drawn into a pipette and form droplets that do not appear clumped or stringy when discharged from the pipette.
- Normal droplets form a thin thread when released from the pipette.
- Droplets with threads longer that 2 centimeters are considered highly viscous, usually from incompletely liquefied specimens
- Increased viscosity and incomplete liquefaction impede sperm motility.
- Ratings of 0 (watery) to 4 (gel-like) can be assigned to the viscosity report. Viscosity can also be reported as low, normal, and high.
pH
- This is tested by applying semen to a pH pad of a urinalysis reagent strip and then the resulting color is compared with the manufacturer’s chart. Dedicated pH testing paper also can be used.
- The normal pH of semen is alkaline with a range of 7.2 to 8.0.
- Increased pH is indicative of infection within the reproductive tract.
- A decreased pH is associated with increased prostatic fluid.
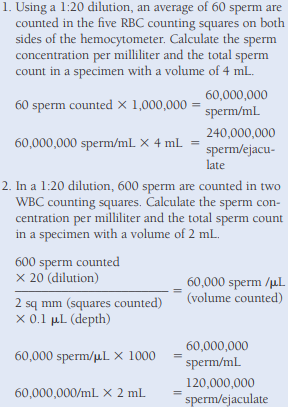
Sperm Concentration/Count
The recommended method is the Neubauer chamber count.
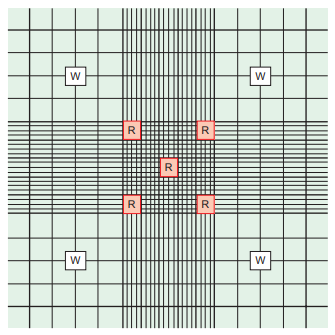
- The dilution used is a 1:20 sodium bicarbonate and formalin or saline and distilled water solution prepared using a mechanical (positive-displacement) pipette to immobilize and preserve the cells.
- Counts are performed using either phase or bright-field microscopy, and the addition of stain, such as crystal violet, to the diluting fluid aids in visualization when using bright-field microscopy.
- Sperm are usually counted in the four corner and center squares of the large center square.
- Only fully developed sperm should be counted.
- Immature sperm and WBCs, often referred to as “round” cells, are able to be differentiated by the stain. They must not be included but should be noted and counted separately.
- The presence of more than 1 million spermatids per milliliter indictes disruption of spermatogenesis. This may be caused by viral infections, exposure to toxic chemicals, and genetic disorders.
- Greater than 1 million leukocytes per milliliter is associated with inflammation or infection of the reproductive organs that can lead to infertility.
The counts should agree within 10%, and the average of the two counts is used in the calculation.
- If the counts do not agree, both the dilution and the counts are repeated.
Sperm Motility
- The presence of sperm capable of forward, progressive movement is critical for fertility, because once presented to the cervix, the sperm must propel themselves through the cervical mucosa to the uterus, fallopian tubes, and ovum.
- Assessment of sperm motility should be performed on well mixed, liquefied semen within 1 hour of specimen collection. To provide continuity in reporting, laboratories should place a consistent amount of semen under the same size coverslip, such as 10 μL under a 22 x 22 mm coverslip.
- Motility is evaluated by both speed and direction. Grading can be done using a numerical or alphabetical scale.
- 50% or more sperm should be motile with a rating of 2.0 or be in categories a, b, and c after 1 hour. 25% in progressive motility (categories a and b) is also acceptable.
| Numerical Grade | Alphabetical Grade | WHO Criteria |
|---|---|---|
| 4.0 | a | rapid, straight-line motility |
| 3.0 | b | slower speed, some lateral movement |
| 2.0 | b | slow forward progression, noticeable lateral movement |
| 1.0 | c | no forward progression |
| 0 | d | no movement |
- The presence of a high percentage of immobile sperm and clumps of sperm requires further evaluation to determine sperm viability or the presence of sperm agglutinins.
- In recent years, instrumentation capable of performing computer-assisted semen analysis (CASA) has been developed
- CASA provides objective determination of both sperm velocity, trajectory, sperm concentration and morphology.
- Currently, CASA instrumentation is found primarily in laboratories that specialize in andrology and perform a high volume of semen analysis.
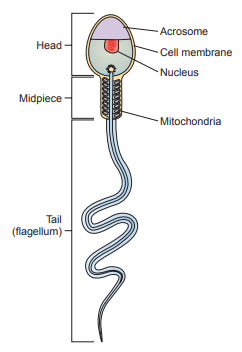
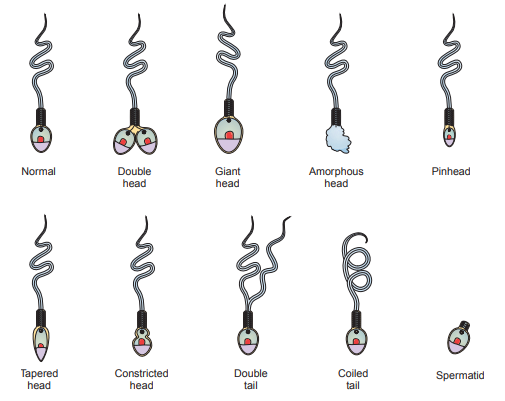
Sperm Morphology
Sperm morphology is evaluated with respect to the structure of the head, neckpiece, midpiece, and tail.
- Abnormalities in head morphology are associated with poor ovum penetration.
- Neckpiece, midpiece, and tail abnormalities affect motility.
The normal sperm has an oval-shaped head approximately 5 μm long and 3 μm wide and a long, flagellar tail approximately 45 μm long.
The enzyme-containing acrosomal cap located at the tip of the head is critical for ovum penetration.
The acrosomal cap should encompass approximately half of the head and covers appproximately two-thirds of the sperm nucleus.
The neckpiece attaches the head to the tail and the midpiece.
The midpiece is the thickest part of the tail because it is surrounded by a mitochondrial sheath that produces the energy required by the tail for motility.
Sperm morphology is evaluated from a thinly smeared, stained slide using Wright’s, Giemsa, or Papanicolaou stain under oil immersion and are stable for 24 hours.
At least 200 sperm should be evaluated and the percentage of abnormal sperm reported.
Abnormalities in head structure include double heads, giant and amorphous heads, pinheads, tapered heads, and constricted heads.
Abnormal sperm tails are frequently doubled, coiled, or bent.
An abnormally long neckpiece may cause the sperm head to bend backward and interfere with motility.
Additional parameters include measurement of head, neck, and tail size, size of the acrosome, and the presence of vacuoles and require the use of a stage micrometer or morphometry; however, it is not required in routine examinations but recommended by the WHO.
Normal values for sperm morphology vary from greater than 30% normal forms when using routine criteria to greater than 14% normal forms when using strict criteria.
For round cells, they are calculated using the formula C = (N x S)/100, where N equals the number of spermatids or neutrophils counted per 100 mature sperm, and S equals the sperm concentration in millions per milliliter.
- This method can be used when counting cannot be performed during the hemocytometer count and to verify counts performed by hemocytometer.
Additional Testing
Sperm Viability
- Viability is evaluated by mixing the specimen with an eosin-nigrosin stain, preparing a smear, and counting the number of dead cells in 100 sperm.
- Living cells are not infiltrated by the dye and remain a bluish white color, whereas dead cells stain red against the purple background.
- Normal viability requires 75% living cells and should correspond to the previously evaluated motility.
- Decreased sperm viability may be suspected when a specimen has a normal sperm concentration with markedly decreased motility.
Seminal Fluid Fructose
Specimens can be screened for the presence of fructose using the resorcinol test that produces an orange color when fructose is present.
- A reagent of 50 mg resorcinol in 33 mL concentrated HCl diluted to 100 mL with water is prepared.
- 9 mL of it is mixed with 1 mL of semen.
- It is boiled until an orange-red color is observed.
Quantitatively, it can be determined using spectrophotometric methods.
- A normal quantitative level of fructose is equal to or greater than 13 μmol per ejaculate.
Specimens for fructose levels should be tested within 2 hours or frozen to prevent fructolysis.
Low to absent fructose levels indicate a low sperm concentration caused by lack of the support medium produced in the seminal vesicles.
Antisperm Antibodies
- Antisperm antibodies can be present in both men and women and are detected in semen, cervical mucosa, or serum.
- It is not unusual for both partners to demonstrate antibodies, although male antisperm antibodies are more frequently encountered.
- In males, the blood-testes barrier separates sperm from the male immune system, but when this barrier is disrupted due to surgery, trauma and infection, the antigens on the sperm produce an immune response that damages the sperm.
- The damaged sperm may cause the production of antibodies in the female partner.
- The presence of antibodies can be suspected when clumps of sperm are observed during a routine semen analysis for males, and when the semen agglutinates with cervical mucosa despite a normal semen analysis for females.
- Two frequently used tests to detect the presence of antibody-coated sperm are the mixed agglutination reaction (MAR) test and the immunobead test.
- The MAR test is a screening procedure used primarily to detect the presence of immunoglobulin G (IgG) antibodies.
- The semen sample containing motile sperm is incubated with IgG antihuman globulin (AHG) and a suspension of latex particles or treated RBCs coated with IgG.
- The bivalent AHG binds simultaneously to both the antibody on the sperm and the antibody on the latex particles or RBCs, forming microscopically visible clumps of sperm and particles or cells.
- Less than 10% of the motile sperm attached to the particles is considered normal.
- The immunobead test is a more specific procedure in that it can be used to detect the presence of IgG, IgM, and IgA antibodies and demonstrates what area of the sperm (head, neckpiece, midpiece, or tail) the autoantibodies are affecting.
- Head-directed antibodies can interfere with penetration into the cervical mucosa or ovum, whereas tail-directed antibodies affect movement through the cervical mucosa.
- In the immunobead test, sperm are mixed with polyacrylamide beads known to be coated with either antiIgG, anti-IgM, or anti-IgA.
- The presence of beads on less than 20% of the sperm is considered normal.
Microbial and Chemical Testing
- The presence of more than 1 million leukocytes per millimeter indicates infection within the reproductive system, frequently the prostate.
- Routine aerobic and anaerobic cultures and tests for Chlamydia trachomatis, Mycoplasma hominis, and Ureaplasma urealyticum are most frequently performed.
- Additional chemical testing performed on semen may include determination of the levels of neutral α-glucosidase, zinc, citric acid, and prostatic acid phosphatase.
- Decreased neutral α-glucosidase suggests a disorder of the epididymis.
- Decreased zinc, citrate, and acid phosphatase indicate a lack of prostatic fluid.
- On certain occasions, the laboratory may be called on to determine whether semen is actually present in a specimen for cases of alleged rape.
- Microscopically examining the specimen for the presence of sperm may be possible, with the best results being obtained by enhancing the specimen with xylene and examining under phase microscopy.
- Seminal fluid contains a high concentration of prostatic acid phosphatase, therefore the detection of this enzyme can aid in determining the presence of semen in a specimen.
- A more specific method is the detection of seminal glycoprotein p30.
- Further, information can often be obtained by performing ABO blood grouping and DNA analysis on the specimen.
| Analyte | Normal Values |
|---|---|
| neutral α-glucosidase | ≥20 mU/ejaculate |
| zinc | ≥2.4 μmol/ejaculate |
| citric acid | ≥52 μmol/ejaculate |
| acid phospatase | ≥200 units/ejaculate |
Post-vasectomy Semen Analysis
The concern is the presence or absence of spermatozoa.
- The length of time required for complete sterilization can vary greatly among patients and depends on both time and number of ejaculations.
Specimens are routinely tested at monthly intervals, beginning at 2 months post-vasectomy and continuing until two consecutive monthly specimens show no spermatozoa.
- Recommended testing includes examination of a wet preparation using phase microscopy for the presence of motile and nonmotile sperm.
- A negative wet preparation is followed by centrifugation of the specimen for 10 minutes and examination of the sediment.
Sperm Function Tests
- The tests are most commonly performed in specialized andrology laboratories and include the hamster egg penetration assay, cervical mucus penetration test, hypo-osmotic swelling test, and the in vitro acrosome reaction.
- In the hamster egg penetration assay, sperm are incubated with species non-specific hamster eggs and penetration is observed microscopically.
- In the cervical mucus penetration test, it evaluates the sperm penetration ability of one’s partner’s midcycle cervical mucus.
- In the hypo-osmotic swelling test, sperm exposed to low-sodium concentrations are evaluated for membrane integrity and sperm viability.
- In the in vitro acrosome reaction, the acrosome is evaluated on its production of enzymes essential for ovum penetration.
Semen Analysis Quality Control
- Traditionally, semen routine analysis has been subject to very little quality control resulting from a lack of appropriate control materials and the subjectivity of the motility and morphology analyses.
- The analysis is rated as a high complexity test under the Clinical Laboratory Improvement Amendments, and testing personnel standards must be observed
- The standardized procedures developed by the WHO have provided a basis for laboratory testing and reporting.
- The use of CASA has aided in reducing the subjectivity of the analysis, though it has still been shown to vary among operators.
- Laboratories can now participate in proficiency testing programs offered by the College of American Pathologists and the American Association of Bioanalysts (AAB) that include sperm concentration, viability, and morphology.
- Commercial quality control materials and training aids are available and should be incorporated into laboratory protocols.