IB11 Chemistry HL
Preliminary stuff:
Equations:


c=fλ
E=hf
n=m/M
n=CV
PV=nRT
(P1V1)/T1=(P2V2)/T2
Q=mcΔT
% atom economy=(molar mass of desired product)/(molar mass of all reactants)×100
ΔH⦵=Σ(ΔHf⦵products)−Σ(ΔHf⦵reactants)
ΔH⦵=Σ(ΔHc⦵reactants)−Σ(ΔHc⦵products)
ΔG⦵=ΔH⦵−TΔS⦵
ΔG=ΔG⦵+RTlnQ
ΔG⦵=−RTlnK
ΔG⦵=−nFE⦵
k=Ae^(−Ea/RT)
lnk=(−Ea)/(RT)+lnA
pH=−log10[H3O+] or pH=−log10[H+]
Kw=[H+][OH–]
pOH=−log10[OH–]
Physical constants:
e: Elementary charge. 1.602177×10^−19 C
me: Electron rest mass: 9.109384×10^−31 kg
mp: Proton rest mass. 1.672622×10^−27 kg
mn: Neutron rest mass. 1.674927×10−27 kg
c: Speed of light in a vacuum. 3.00×108 ms^−1
h: Planck constant. 6.63×10^−34 J s
NA: Avogadro’s constant. 6.02×10^23 mol−1
R: Gas constant. 8.31 J K^−1 mol^−1
VM: Molar volume of an ideal gas at STP. 2.27×10^−2 m^3 mol^−1=22.7 dm^3 mol^−1
cW: Specific heat capacity of water. 4.18 kJ kg^−1 K^−1=4.18 J g^−1 K^−1
KW: Ionic product constant for water at 298.15 K. 1.00×10^−14 mol^2 dm^−6
F: Faraday constant. 9.65×104 C mol^−1
Metric (SI) multipliers:
peta | P | 10^15 |
|---|---|---|
tera | T | 10^12 |
giga | G | 10^9 |
mega | M | 10^6 |
kilo | k | 10^3 |
hecto | h | 10^2 |
deca | da | 10^1 |
deci | d | 10^−1 |
centi | c | 10^−2 |
milli | m | 10^−3 |
micro | μ | 10^−6 |
nano | n | 10^−9 |
pico | p | 10^−12 |
femto | f | 10^−15 |
Unit conversions:
Temperature (K) = temperature (°C) + 273.15
1 dm^3 = 1 litre = 1 × 10^−3 m3 = 1 × 10^3 cm3
STP conditions: 273.15 K and 100 kPa
SATP conditions: 298.15 K and 100 kPa
Electromagnetic spectrum:
Elements, compounds, and mixtures:
Vocabulary:
Pure substances: made up of one type of substance and have a fixed composition.
Mixtures: made by combining two or more pure substances together, so they do not have a fixed composition
Elements: The building blocks of matter. Simplest forms of matter and consist of only one type of atom.
Allotropes: different forms of an element in the same physical state.
Compounds: Composed of two or more different elements chemically combined in fixed ratios.
Homogeneous mixtures: Do not have visible phases or boundaries, and they have a uniform composition, making the composition the same throughout the mixture.
Heterogeneous mixtures: Has visible phases or boundaries and is non-uniform, meaning that different parts of the mixture have a different composition.
Notes:
Mixtures:
One or more elements or compounds in no fixed ratio.
Contain pure substances that are not chemically bonded so they can be separated by physical means.
Components of this retain their individual properties.
Homogenous mixture:
Can be considered homogenous when all substances are in the same state and the same amount.
Separation techniques:
Vocabulary:
Filtration: The separation of an insoluble solid from a liquid or solution
Filtrate: A substance that has passed through a filter.
Residue: The insoluble component, usually a solid, of a mixture that remains after filtration.
Solvation: The process where the solvent particles surround and interact with the particles of the solute.
Solubility: The ability of a substance to dissolve into a solvent to form a solution.
Evaporation: Used to separate a mixture which has a solute dissolved in a solvent which is usually water.
Distillation: The separation of a liquid mixture based on the difference in volatility or boiling points between the components of the mixture
Volatility: The tendency of a substance to undergo evaporation.
Miscible: Capable of being mixed in any ratio without separation.
Paper chromatography: Used to separate a mixture of solutes in a solvent.
Recrystallisation: Used to remove impurities that are mixed in with a solid.
Residue: The insoluble component, usually a solid, of a mixture that remains after filtration.
Condenser: A piece of laboratory glassware used to condense a gas into a liquid using cold water.
Chromatogram: The output from chromatography. In liquid chromatography this is the paper containing the separating mixture.
Synthetic: A substance which is artificially made or produced, usually through chemical reactions in a laboratory or factory.
Dyes: Synthetically made and are typically small, polar molecules. Will dissolve in a polar solvent such as water and produce a transparent solution.
Pigments: Naturally derived from plants and are typically large, non-polar molecules. When mixed with a solvent will remain suspended and create an opaque solution.
Solvent front: The distance from the pencil line to where the solvent (the mobile phase) reached on the chromatography paper at the time it was removed from the solvent.
Notes:
Solvation:
Can separate a heterogenous mixture of solids through differing solubility
When one substance is soluble and the other isn’t, the insoluble solid can be separated with filtration.
Distillation:
Each liquid has a different boiling point.
One evaporates first and rise up the distillation column through the condenser.
Paper chromatography:
The mixture is first dissolved in a solvent. Known as the mobile phase
A piece of chromatography paper is then placed in the solution. Known as the stationary phase.
Components of the mixture move through the stationary phase at different rates due to differences in solvation
Recrystallisation:
The impure mixture is first dissolved in a small volume of hot solvent. Any insoluble impurities can be filtered off.
The solution is then cooled which causes the solubility of the dissolved solids to decrease.
The desired product forms crystals leaving the soluble impurities in the solution
Impurities are then filtered to obtain the pure product.
States of matter and changes of states:
Vocabulary:
Kinetic energy:
Density: Mass per unit volume
Heat energy: A form of energy that is transferred between objects of different temperatures.
Physical change: A reversible change that does not change the chemical properties of a substance.
Sublimation: The change of state from a solid to a gas with no liquid state.
Deposition: The change of state from gas to solid with no liquid state.
Vaporisation: The change of state from a liquid to a vapour (or gas).
Evaporation: a change of state from liquid to gas and takes place only at the surface of the liquid. Can occur at temperatures below the boiling point of the liquid.
Boiling: occurs at a specific temperature and is a change of state from liquid to gas throughout the liquid. Bubbles of gas are formed within the liquid, not only at the surface.
Notes:
Kinetic molecular theory:
All matter is made up of small particles
Particles all have kinetic energy
The amount of kinetic energy is proportional to temperature
Collisions between particles are elastic, meaning no energy is lost.
Solid:
Cannot be compressed because molecules are already packed
Fixed shape and fixed volume due to strong forces of attraction
Solids cannot flow.
Liquid:
There are weaker forces of attraction.
Do not have a fixed shape and usually take the shape of the bottom of its container
Still cannot be compressed
Gas:
Do not have a fixed shape or volume
Takes the same shape as the container
Volume depends on the temperature and the pressure of the gas itself
Very weak attraction
Can be compressed
Temperature and kinetic energy:
Formula:
Ek: 1/2mv^2
Vocabulary:
Celcius: Based on the freezing point of water (0 °C) and the boiling point of water (100 °C).
Kelvin: An absolute temperature scale where the lowest possible value is 0 K, known as absolute zero.
Absolute zero: 0 K. Particles have zero kinetic energy.
Heating curve: Shows how the state of matter changes as heat is added. Opposite is cooling curve
Intermolecular force: Attractive (or repulsive) forces that exist between the molecules of a substance.
Notes:
Celcius and Kelvin:
To get Kelvin from Celcius, add 273, and vice versa.
Heating:
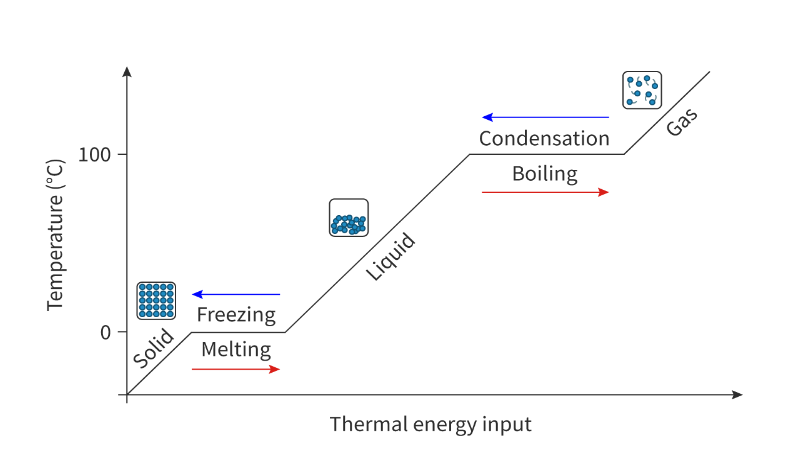
The temperature remains constant during change of state because all the added/removed heat is being used to overcome the intermolecular forces that act between the particles.
The relationship between the temperature in Kelvin and the kinetic energy of the particles is directly proportional.
Atomic structure:
Vocabulary:
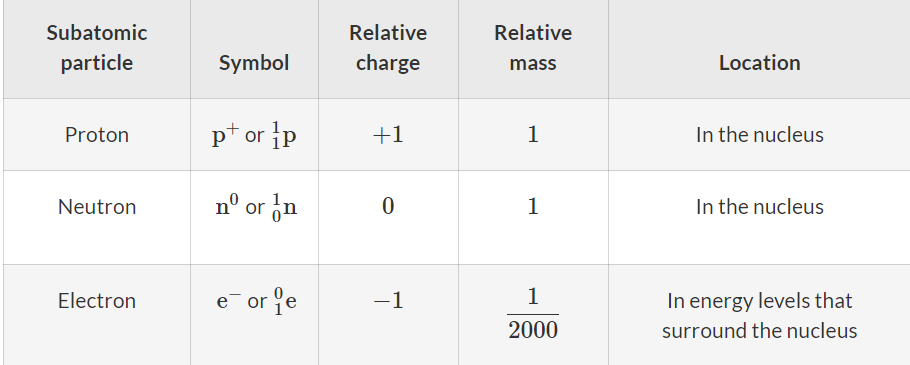
Nucleus: The region at the centre of the atom where the protons and neutrons are located.
Electrons: The subatomic particles with a relative mass of 1/2000 and a charge of −1.
Protons: The subatomic particle with a relative mass of 1 and a charge of +1.
Neutrons: The subatomic particle with a relative mass of 1 and no charge.
Nucleons: A collective term for protons and neutrons.
Atomic number: The number of protons in the nucleus of an atom. Symbol Z.
Mass number: Also known as the nucleon number. The number of protons and neutrons in the nucleus of an atom. Symbol A.
Ions: A charged particle. It has a charge as the number of protons do not equal the number of electrons.
Notes:
Isotopes:
Vocabulary:
Isotopes: atoms of the same element that have different numbers of neutrons.
Relative atomic mass: Atoms of the same element that have different numbers of neutrons.
Percent abundance: The percent of an isotope in a naturally occurring sample of an element
Notes:
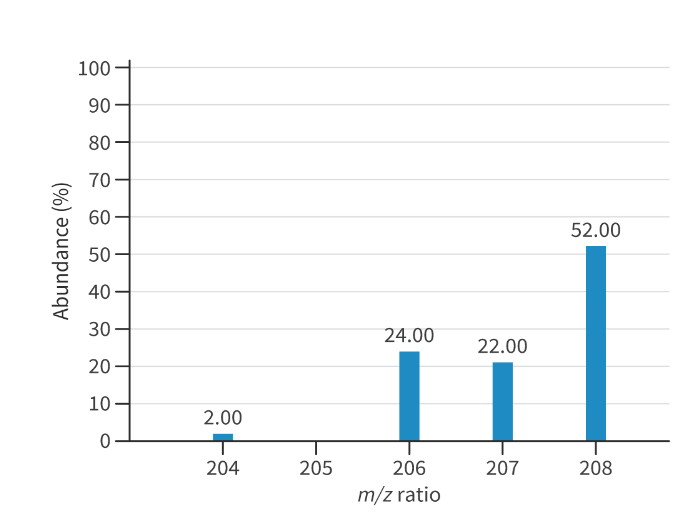
Isotopes:
Different physical properties due to mass (boiling point, melting point, density)
Mass spectra of elements:
Vocabulary:
Mass spectrometer: An analytical device used to determine the m/z ratio of the isotopes in a naturally occurring sample of an element.
m/z ratio: The ratio of the mass of an ion divided by its charge.
Molecular ions: A positively charged ion produced in a mass spectrometer.
Relative intensity: The size of a peak in a mass spectrum relative to the most abundant ion which is shown as the tallest peak in the spectrum.
Base peak: In mass spectrometry, the peak associated to the most abundant ion. It is used to calculate the relative intensity of other ions.
Notes:
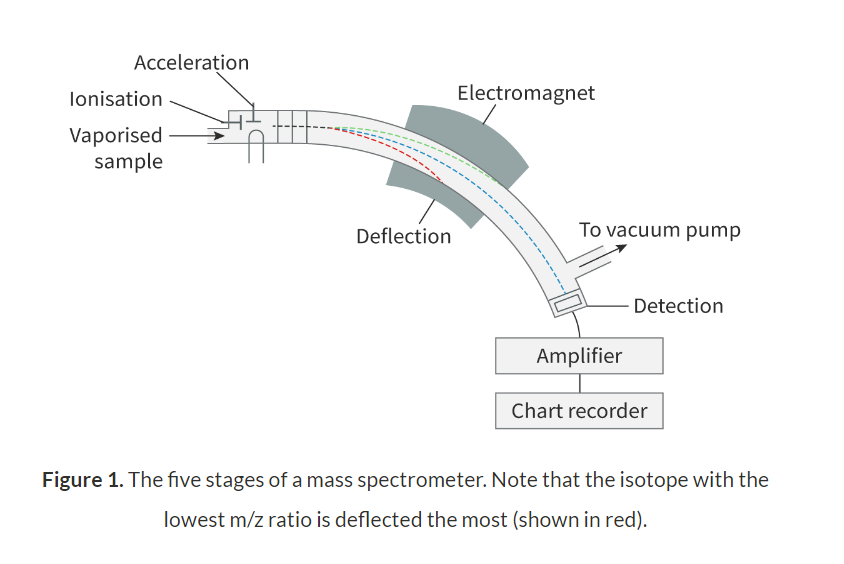
Mass spectrometry:
Vaporisation: The substance is vaporised to produce gaseous molecules.
Ionisation: High-energy electrons are fired at the said gaseous molecules, which causes them to be ionised, forming gaseous ions.
Acceleration: The gaseous ions are accelerated in an electric field.
Deflection: The gaseous ions are deflected by an electromagnet. The degree of deflection they undergo depends on their m/z ratio. Ions with lower m/z ratios are deflected the most and ions with higher m/z ratios are deflected the least.
Detection: the gaseous ions are detected and a mass spectrum is produced.
Mass spectrum graph:
Line spectra:
Vocabulary:
Electromagnetic spectrum: The range of frequencies or wavelengths of electromagnetic radiation.
Visible light: The segment of the electromagnetic spectrum that the human eye can see, typically between 380 to 700 nanometers.
White light: Light that is composed of all the frequencies or wavelengths of visible light.
Spectroscopy: The study of the interaction between electromagnetic radiation and matter.
Emission spectra: The range of frequencies or wavelengths of electromagnetic radiation emitted during an electron transition from a higher to a lower energy level.
Electron transitions: The movement of an electron between the energy levels in an atom, accompanied by the absorption or emission of energy
Frequency v: The number of complete waves passing a point each second. This is measured in hertz (Hz).
Wavelength λ,: The distance from a point on one wave to the equivalent point on the adjacent wave. This is measured in metres (m).
Oscillate: Move or swing back and forth at a regular speed.
Spectroscope: A device for viewing the emission spectra of elements in the visible region.
Emission line spectrum: The range of frequencies or wavelengths of electromagnetic radiation emitted during an electron transition from a higher to a lower energy level.
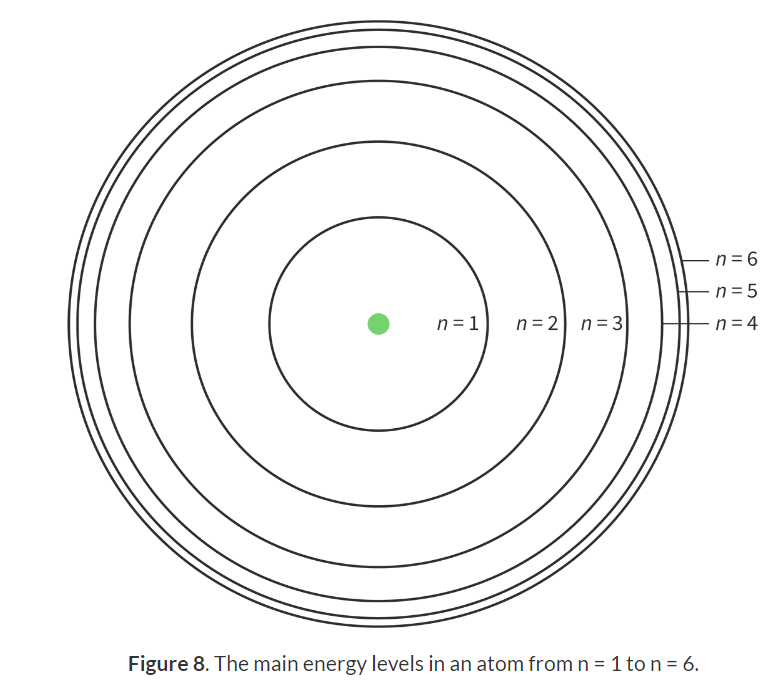
Bohr model of the atom: An atomic model that shows energy levels at fixed distances from the nucleus.
Principal quantum number: The main energy levels occupied by electrons, assigned the letter n.
Ground state: The lowest energy state of an atom when all its electrons occupy their lowest energy levels.
Photons: An elementary particle of discrete amounts of electromagnetic radiation.
Excited state: A higher energy state than the ground state when electron(s) gain energy and move to higher energy levels.
Notes:
Electromagnetic spectrum:
All regions of the electromagnetic travel at the speed of light - 3*10^8 m/s
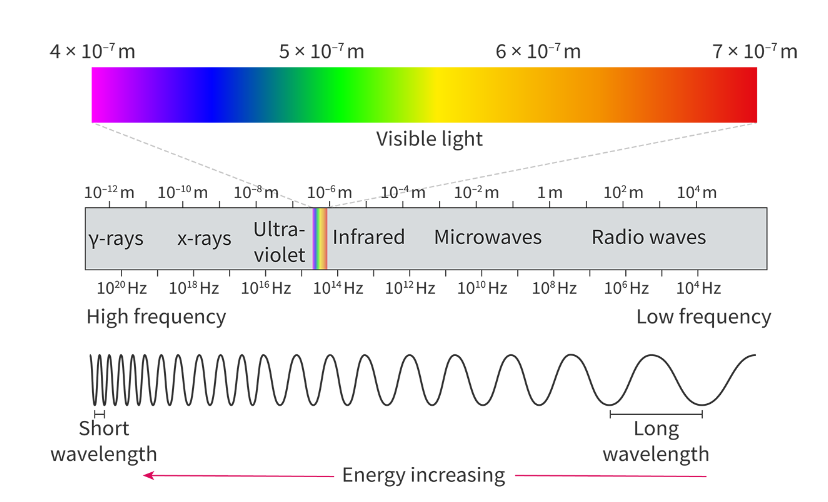
Continuous spectrum:
All wavelengths and frequencies of visible from red to violet.

Emission line spectrum:
Only shows specific wavelengths or frequencies of light
Shown as coloured lines on a black background.
Converge at higher energies due to higher frequnecy and shorter wavelength, v.v.

Bohr model of the atom:
Electrons orbit the nucelus
Electrons can only exist in certain shells, not in between.
Electrons could move between levels by emitting/absorbing energy.
Converge at higher energy levels
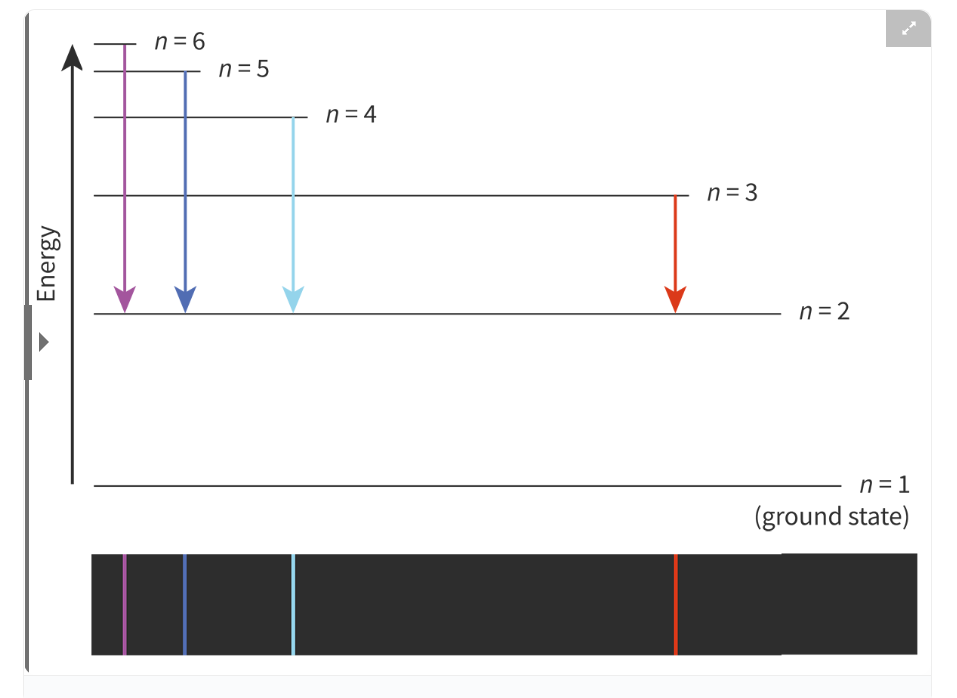
Electron transition:
If an electron absorb a discrete amount of energy, it will transition from a lower to a higher energy level
The unstable electron emits the same amount of energy and transitions back
Transitioning through more levels require/generate more energy, which is indicated by the colour.
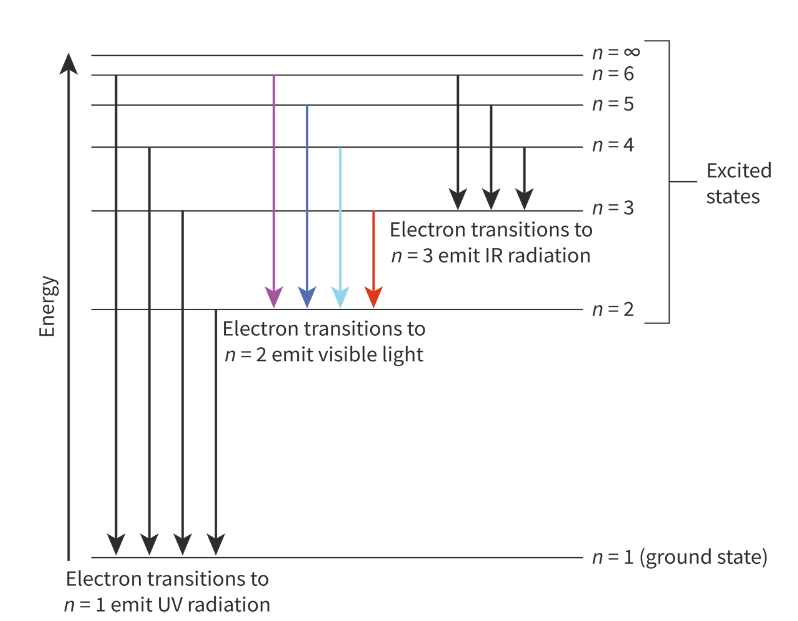
Hydrogen emission spectrum:
From higher energy levels to n = 1 emit energy corresponds to UV radiation. Highest energy transitions.
From higher energy levels to n = 2 emit energy corresponds to visible light.
From higher energy levels to n = 3 emit energy corresponds to infrared radiation. Lowest energy transitions.
 Main energy levels and sub-levels:
Main energy levels and sub-levels:
Vocabulary:
Heisenberg uncertainty principle: A scientific principle that states we cannot know both exact location and velocity of an electron at the same time.
Sub-levels: The smaller division of the main energy levels, assigned the letters s, p, d or f.
Notes:
Main and sub levels:
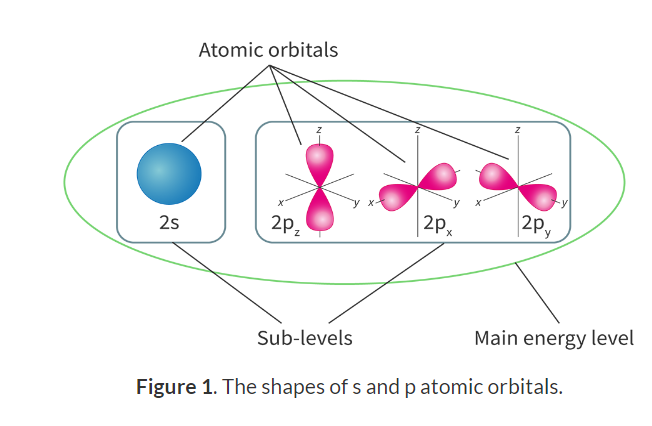
Electron configuration:
Vocabulary:
Electron configuration: Electronic configuration shows the arrangement of electrons in their different levels around the nucleus of an atom.
Aufbau principle: A scientific principle that states that electrons fill the atomic orbital of the lowest energy levels first.
Pauli exclusion principle: An atomic orbital can only hold two electrons and they must have opposite spins.
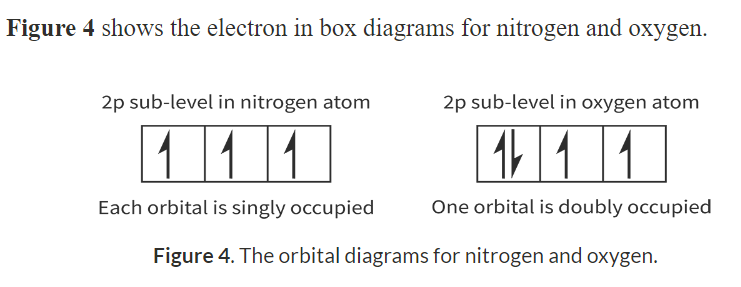
Hund’s rule: when we have degenerate orbitals then each orbital is filled with a single electron before being doubly occupied.
Degenerate orbitals: Atomic orbitals that have equal energy levels. For example, the three 3p orbitals are degenerate orbitals.
D-block elements: Elements located in groups 3–12 of the periodic table which have their valence electrons in the d-orbitals.
Orbital diagrams (Arrow-in-box diagrams): Diagrams that represent how electrons occupy the atomic orbitals of an atom. Drawn as square boxes with headed arrows to show spin directions.
Notes:
Sub-levels order:
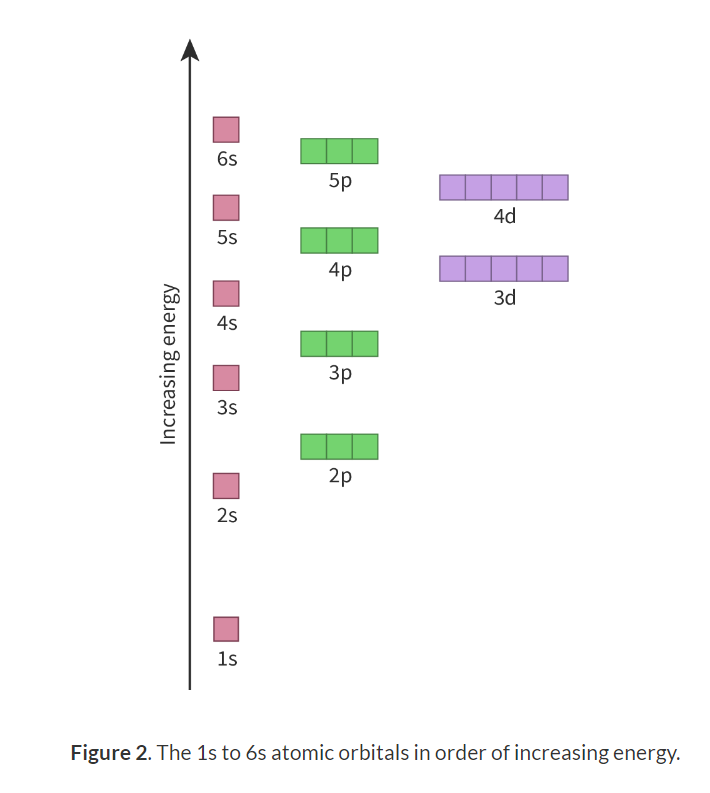
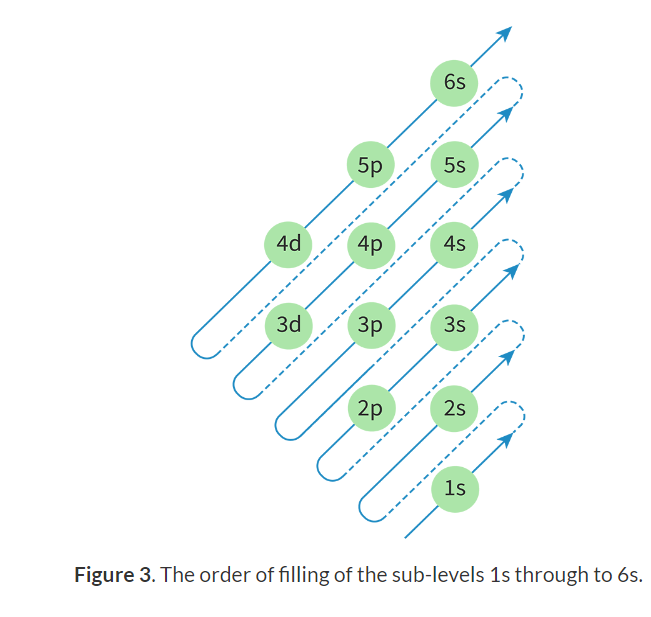 Condensed electron configuration:
Condensed electron configuration:
Write full configuration
Write the symbol of the previous noble gas.
Write for each sub-level (s, p, d, f) their largest variant.
E.g. Carbon = 1s^2, 2s^2, 2p^2 = He 2s^2. 2p^2
Exceptions of Aufbau principle:
Cr (Chromium) = Ar 4s^1, 3d^5
Cu (Copper) = Ar 4s^2, 3d^10
Orbital diagrams:
Only two arrows in one box.
Must have opposite spins
Must form all singles first before forming doubles
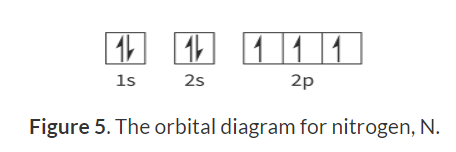
Calculating ionisation energies:
Formula:
E = hf
E: energy. Joules (J)
h: Planck’s constant, 6.63*10^-34. Joule seconds (J s)
f: frequency: seconds (s^-1)
c = λf:
c: Speed of light, 3.00*10^8. Meters per second (m/s)
λ: wavelength. Meters (m)
Vocabulary:
Strong nuclear force: The force that attracts subatomic particles, such as protons and neutrons, toward each other.
Ionisation energy: The energy required with remove one mole of electrons from one mole of gaseous atoms to form one mole of gaseous 1+ ions.
Convergence line: The point at which the spectral lines converge. Can be used to calculate the ionisation energy.
Avogadro’s number: The number of particles in one mole of substance. Equal to 6.02 × 1023
Notes:
Ionisation:
Removing an electron in its ground state. E.g. Hydrogen, from n=1 to n=∞
The energy required is dependent on the nuclear charge (# of protons) and the distance between the nucleus and the outer electrons.
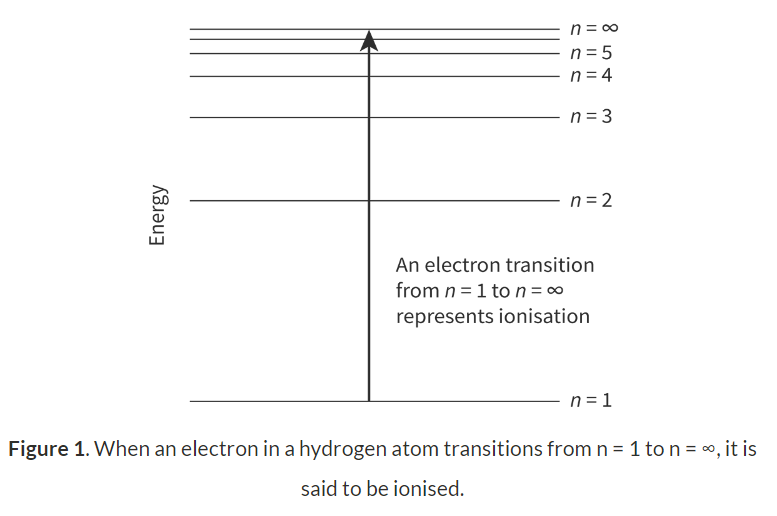
Trends in ionisation:
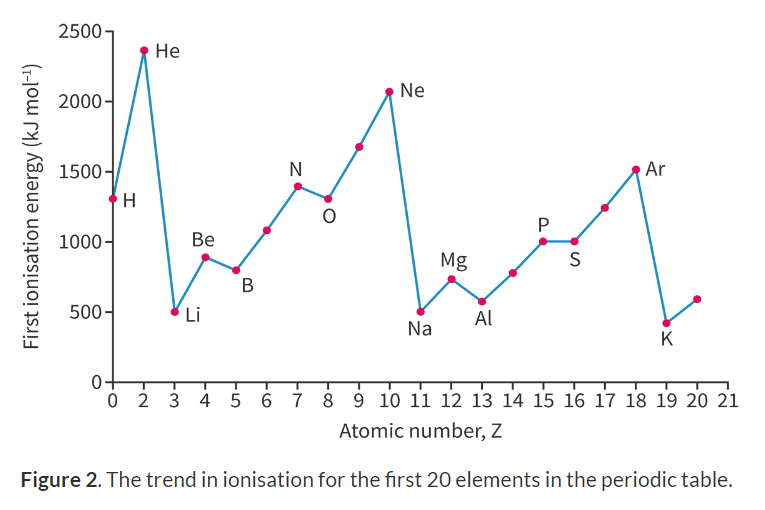 Beryllium and Boron:
Beryllium and Boron:
Beryllium has the electron configuration 1s^2 2s^2
Boron has the electron configuration 1s^2 2s^2 2p^1
Electrons of the p-energy levels are further from the nucleus than s-energy levels and thus are easier to remove.
The same goes of Magnesium (Mg) and Aluminium (Al)
Nitrogen (N) and Oxygen (O):
Nitrogen has the electron configuration 1s^2 2s^2 2p^3
Oxygen has the electron configuration 1s^2 2s^2 2p^4
The first doubly occupied electron is repulsed by the second doubly occupied electron and requries less energy to remove.
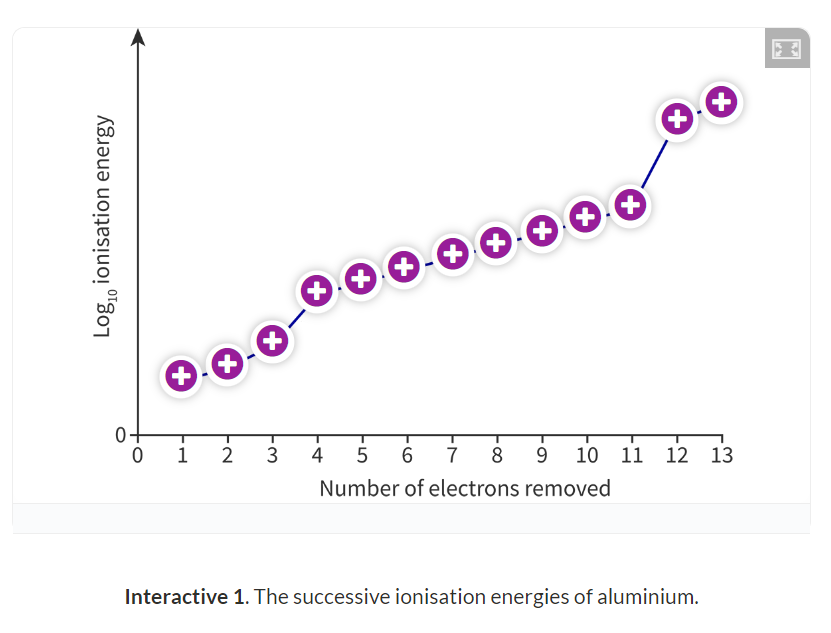
Successive ionisation energies:
Vocabulary:
Successive ionisation energies: The energies required to remove more and more electrons from an ion that is becoming increasing positive.
Notes:
Successive ionisation energies:
Progressively need more energy because the ion is becoming increasinly more positive.
This leads to an increase in the electrostatic attraction between the nucleus and the remaining electrons.
There is a sharp increase in energy whenever it switches to a different energy level, which can be used to determine the number of valence electrons.
The mole and Avogadro’s constant:
Formula:
Molar mass: n = m/M
n is “moles”
m is “mass”
M is “molar mass”
Vocabulary:
Mole: The number of particles present in exactly 12 g of the carbon-12 isotope. This is equal to the Avagadro constant: 6.02 × 1023 particles.
Elementary entities: Any chemical particle such as atoms, molecules, ions or electrons.
Formula unit: The empirical formula for an ionic compound that represents the simplest ratio of ions making up the compound.
Relative atomic mass: The weighted average mass of an atom compared to 1/12 the mass of an atom of carbon-12.
Relative formula mass: The mass of a compound relative to 1/12 the mass of an atom of carbon-12. Does not have any units.
Molar mass: The mass of one mole of a substance, expressed in units of g mol-1.
Empirical formula:
Law of Definite Proportions: For any given compound, the ratio of constituent elements is fixed. Also known as law of definite composition or Proust's law.
Empirical formula: a chemical formula showing the simplest ratio of elements in a compound rather than the total number of atoms in the molecule.
Molar concentration:
Formulas:
One litre (L) = One cubic decimeter (dm³)
Concentration: C = m/V
C: Concentration (g/dm³)
m: Mass of solute (g)
V: Total volume of solution (dm³)
Molarity: M = mol/V
M: Molarity
mol: Moles of solute
V: Total volume of solution (dm³)
Vocabulary:
Concentration: The number of particles or moles in a given volume, mol dm-3.
Mass balance: Laboratory apparatus used to accurately measure the mass of a substance.
Volumetric flash: A piece of laboratory glassware used to prepare a standard solution with a precise volume.
Notes:
[]:
Square brackets [] is often used to express molar concentration:
e.g, [NaCl] = 1.00 mol/dm³
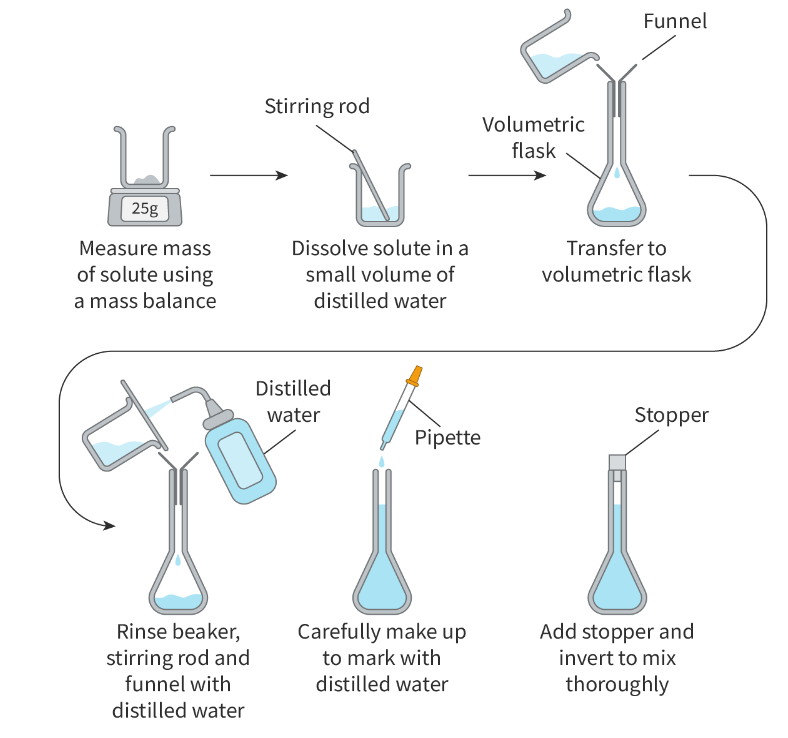
Preparing a standard solution:
Avogadro’s law of combining volumes:
Vocabulary:
Pressure: A measure of the force which the particles in a container exert on the surface as the particles collide with it.
Avogadro’s Law: Equal volumes of gases at the same temperature and pressure will contain the same number of gas particles.
Notes:
Factors affecting gases:
Volume of the container
Temperature of the gas
Pressure of the gas
Ideal gas:
Vocabulary:
Ideal gas: Gases that are assumed to consist of particles that have negligible volume and negligible attractive forces.
Real gas: nonideal gases whose molecules occupy space and have interactions. do not adhere to ideal gas law
Notes:
Ideal gas model:
Particles are in constant, random, and straight-line motion.
Negligible intermolecular forces
Gas volume is negligible; distance between particles is greater than size of particles.
Kinetic energy is directly proportional to absolute temperature (Kelvin).
Any collisions made by particles are elastic, meaning that they just bounce off without losing energy.
Requires high temperature and low pressure
Measurements
Volume:
mL or cm³
L or dm³
Temperature:
Kelvin (K)
Pressure
How frequently particles hit the walls of the container
Pascals (Pa or kPa)