osmosis
Osmosis
Osmosis is the movement of water molecules through a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration, aiming to equalize solute concentrations on both sides of the membrane.
Diffusion
Diffusion is the process by which molecules move from an area of higher concentration to an area of lower concentration until equilibrium is reached. Example: The movement of oxygen from the lungs into the bloodstream.
Active Transport
Active transport is a biological process in which cells move molecules across their membrane against their concentration gradient, requiring energy, typically in the form of ATP. Example: The sodium-potassium pump, which transports sodium ions out of cells and potassium ions into cells, maintaining the electrochemical gradient essential for nerve impulses and muscle contractions.
Partially Penetrable Membrane
A partially penetrable membrane, also known as a semipermeable membrane, allows certain molecules to pass through while blocking others. Example: The cell membrane, which allows water and small nonpolar molecules to pass through but restricts the movement of larger or charged particles.
Concentration Gradient
A concentration gradient is the difference in concentration of a substance between two areas. This gradient typically drives movement from high concentration to low concentration. Example: The gradient of glucose concentration from the bloodstream into the cells, where glucose is utilized for energy.
Methods to Investigate the Effect of Osmosis
Potato Osmosis Experiment: Cut potato tubers into equal-sized pieces and immerse them in various concentrations of salt or sugar solutions. Measure the change in mass before and after immersion to determine how osmosis affects the potato cells.
Dialysis Bag Experiment: Use a dialysis bag filled with a sucrose solution and immerse it in distilled water. Measure the change in volume of the sugar solution inside the bag over time to observe water movement due to osmosis.
Egg Osmosis Experiment: De-shelled eggs can be placed in different solutions (hypotonic and hypertonic). After a set period, measure the change in size and weight of the egg to analyze osmotic effects.
Elodea Leaf Experiment: Place Elodea leaves in freshwater and saltwater environments. Observe the changes in turgor pressure and cell structure under a microscope to see how osmosis affects plant cells in different solutions.
The rate of diffusion is affected by several factors:
Surface Area: The larger the surface area available for diffusion, the faster the molecules can move across it. More area means more room for molecules to enter or leave.
Temperature: Higher temperatures make molecules move faster. When it's warm, molecules have more energy, which speeds up diffusion.
Pressure: Increased pressure can push molecules closer together, which can boost the rate of diffusion. When molecules are packed tightly, they tend to move out into areas of lower concentration more quickly.
Concentration Gradient: A steeper concentration gradient (big difference between high and low concentration) means faster diffusion. Molecules naturally move from areas where there are a lot of them to areas where there are fewer.
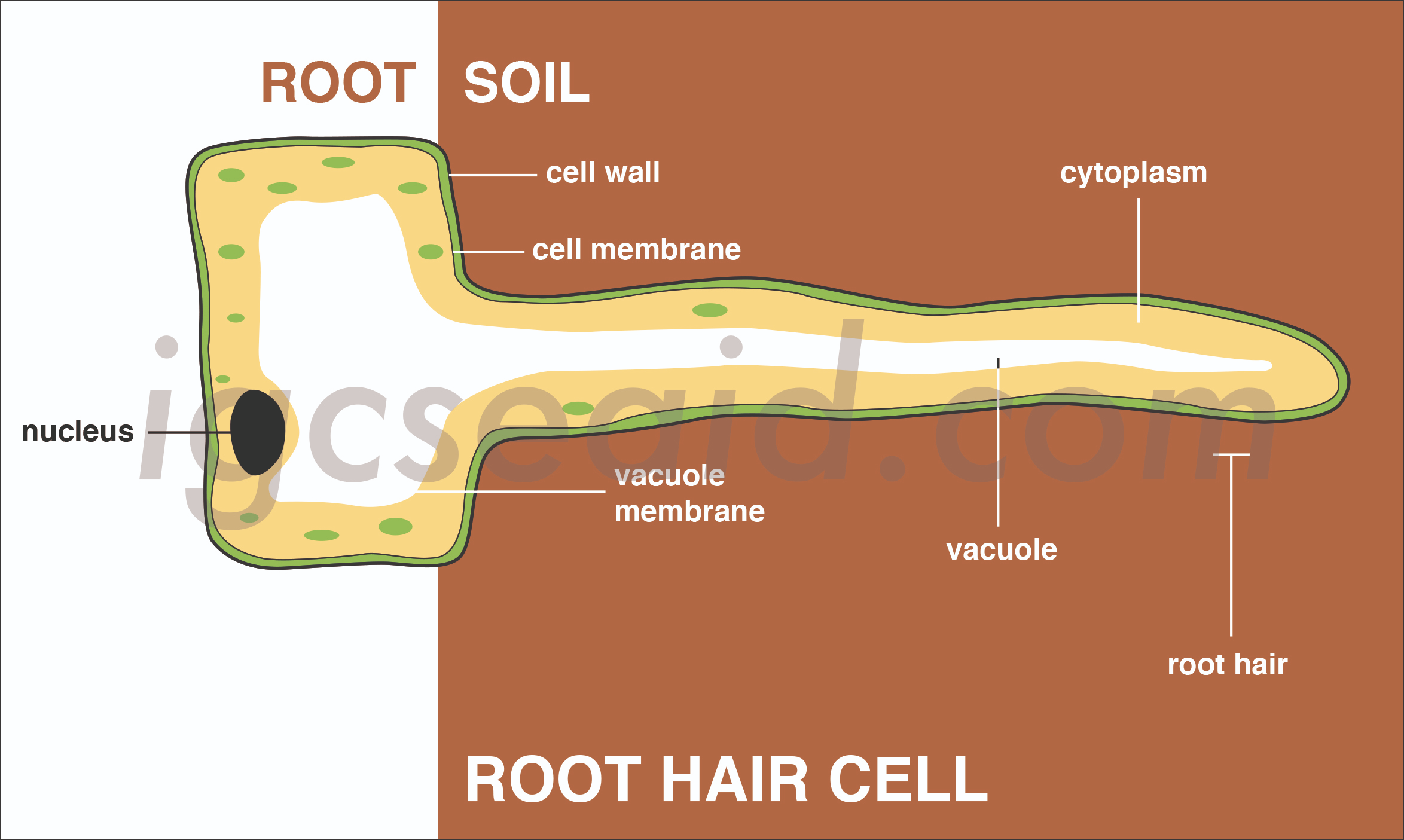
Root hair cells absorb mineral ions from the soil using active transport. Here's how it works:
Active Transport Process: Root hair cells have specialized proteins in their membrane that help pump mineral ions from the soil into the cell against their concentration gradient. This means they can absorb minerals even if the concentration of these minerals is higher inside the cell than in the soil.
Energy Requirement: This process requires energy, which is typically supplied by ATP. The energy is used to move ions from an area of lower concentration (soil) to an area of higher concentration (inside the cell).
Microstructure: The root hair cells have a large surface area due to their long, thin shape, which increases their ability to absorb water and nutrients effectively.