Radioisotopes
Radioisotopes are isotopes of an atom that are unstable and prone to radioactive decay.
Radioactive Decay (overlaps with bio, chem and physics):
Radioactive decay occurs spontaneously when an unstable nucleus is present. There are three types of decay:
Alpha - It happens when there are too many particles crammed in the nucleus. The atom releases an Alpha particle, which consists of two protons and two neutrons. This means that the element changes because the atomic number is now 2 less, and the mass number decreases by 4. The Alpha particle (a Helium nucleus) has a mass of 4, travels slowly compared to other forms of decay and can be stopped by paper or human skin. It is harmful when consumed.
Beta - It happens because there are too many protons compared to neutrons. The atom emits a beta particle, which is a high-speed electron and a proton is converted to a neutron. This means the mass number stays the same, but the atomic number decreases by 1. Beta particles have a negligible (1/2000) mass and travel very fast. They can penetrate human skin and cause damage, but can be stopped by wood, plastic and aluminium.
Gamma - It happens because there is too much energy in the nucleus. Energy is lost from the nucleus in the form of Gamma rays (electromagnetic radiation). This means that no structural changes occur. Gamma rays are the most harmful type of radioactive decay and can only be stopped by thick slabs of lead or concrete.
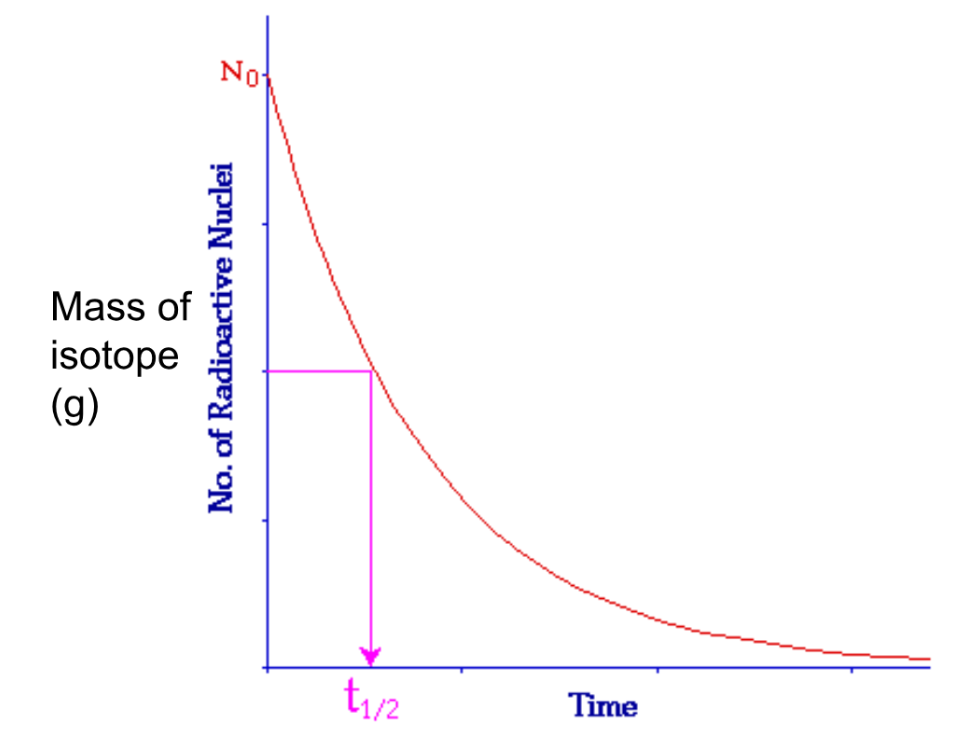
Half-life (2024 notes)
Half-lives are a way of measuring the rate at which the nucleus decays. It is the time it takes for half of the nucleus to decay.
Carbon Dating (briefly covered):
This is a method of dating objects that involves testing carbon isotopes in the object to compare the quantities of each carbon isotope. This ratio helps scientists determine the age of an object.