
Temperature and Thermometers
Temperature:
The measure of the hotness or coldness of a body.
Heat:
A form of energy.
Thermometer:
An instrument used to measure temperature.
Internal Energy
The energy possessed by a body due to the random vibration of its molecules is called internal energy. The higher the temperature of the body the greater the vibrational energy of its molecules is and hence the greater its internal energy.
SI unit of temperature is kelvin (K).
Everyday practical scale of temperature is celsius.
Why do we use kelvin and not celsius?
Kelvin starts at absolute zero, which is equal to -273.15 degrees celsius. Since celsius measures the melting point of ice and boiling point of water, it is less suitable than kelvin as kelvin starts at the number at which physics breaks down, which means in calculations kelvin will never be negative. Hence, we use kelvin.
TC = TK - 273.15
TK = TC + 273.15
Thermometric Properties
Any physical property that changes measurably with temperature is called a thermometric property.
Length of a Column of Liquid
When a liquid is heated it expands. If the liquid is in a thin capillary tube the length of the column of liquid increases as the temperature increases.
This is the thermometric property on which the mercury in glass thermometer and alcohol thermometer depend.
Resistance and Resistance Thermometer
The electrical resistance of a conductor changes when the temperature does. The variation of this resistance with temperature can be measured.
Resistance is the thermometric property on which the resistance thermometer is based.
EMF and a Thermocouple
If two different metals are joined together to form a circuit and the two junctions are maintained at two different junctions, a small EMF (a voltage) appears in the circuit.
The EMF can be measured with a voltmeter. The size of the EMF varies with the temperature difference between junctions.
This device is called a thermocouple and can be used to measure temperature.
Colour Thermometers
The colour of certain crystals changes as the temperature does. This is the basis of one form of thermometer used to measure body temperature.
Volume of a Gas at Constant Pressure
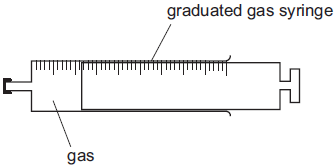
The diagram shows a glass syringe. It contains a fixed mass of gas and a rubber cap seals one end. Atmospheric pressure acts on the piston at the other end; thus the gas is at constant pressure.
If the temperature of the gas is increased, the volume of the gas increases and pushes the piston out.
Temperature and Thermometers
Temperature:
The measure of the hotness or coldness of a body.
Heat:
A form of energy.
Thermometer:
An instrument used to measure temperature.
Internal Energy
The energy possessed by a body due to the random vibration of its molecules is called internal energy. The higher the temperature of the body the greater the vibrational energy of its molecules is and hence the greater its internal energy.
SI unit of temperature is kelvin (K).
Everyday practical scale of temperature is celsius.
Why do we use kelvin and not celsius?
Kelvin starts at absolute zero, which is equal to -273.15 degrees celsius. Since celsius measures the melting point of ice and boiling point of water, it is less suitable than kelvin as kelvin starts at the number at which physics breaks down, which means in calculations kelvin will never be negative. Hence, we use kelvin.
TC = TK - 273.15
TK = TC + 273.15
Thermometric Properties
Any physical property that changes measurably with temperature is called a thermometric property.
Length of a Column of Liquid
When a liquid is heated it expands. If the liquid is in a thin capillary tube the length of the column of liquid increases as the temperature increases.
This is the thermometric property on which the mercury in glass thermometer and alcohol thermometer depend.
Resistance and Resistance Thermometer
The electrical resistance of a conductor changes when the temperature does. The variation of this resistance with temperature can be measured.
Resistance is the thermometric property on which the resistance thermometer is based.
EMF and a Thermocouple
If two different metals are joined together to form a circuit and the two junctions are maintained at two different junctions, a small EMF (a voltage) appears in the circuit.
The EMF can be measured with a voltmeter. The size of the EMF varies with the temperature difference between junctions.
This device is called a thermocouple and can be used to measure temperature.
Colour Thermometers
The colour of certain crystals changes as the temperature does. This is the basis of one form of thermometer used to measure body temperature.
Volume of a Gas at Constant Pressure

The diagram shows a glass syringe. It contains a fixed mass of gas and a rubber cap seals one end. Atmospheric pressure acts on the piston at the other end; thus the gas is at constant pressure.
If the temperature of the gas is increased, the volume of the gas increases and pushes the piston out.
 Knowt
Knowt