Chapter 13 - The Properties of Mixtures: Solutions and Colloids
A solution is a homogenous combination of two or more substances; it exists as a single phase. It is made up of two parts: the solvent and the other chemicals that are dissolved in it.
In general, the solvent is the most abundant component of the solution, whereas the solute is the material that dissolves in the solvent. A solution can contain a variety of solutes.
Typically, the physical state of the solvent dictates the physical state of the solution.
Solutions can be gaseous, liquid, or solid, although this chapter focuses mostly on liquid solutions since they are by far the most significant.
When an excess of a solute is present, its solubility (S) is defined as the greatest amount that dissolves in a constant quantity of a given solvent at a particular temperature. Different solutes have varying degrees of solubility:
- Sodium chloride (NaCl), S = 39.12 g/100. mL water at 100.°C
- Silver chloride (AgCl), S = 0.0021 g/100. mL water at 100.°C
Dipole–induced dipole forces, which are similarly based on polarizability, occur when a polar molecule bends a nonpolar molecule's electron cloud.
They are weaker than ion-induced dipole forces because each pole's charge is smaller than that of an ion (Coulomb's equation).
While the solubility of atmospheric O2, N2, and noble gases in water is restricted, it is owing in part to these factors. They are also used in paint thinners and grease solvents.
Dispersion forces contribute to the solubility of all solutes in all solvents, but they are the primary intermolecular force in nonpolar solutions like petroleum and gasoline. The same forces keep biological macromolecules in their forms.
According to the like-dissolves-like rule, when the forces inside the solute are comparable to those within the solvent, the forces can replace each other and a solution develops. Thus, salts dissolve in water because the ion-dipole attractions between ions and water are similar in intensity to the strong ion-dipole attractions and the strong H bonds between water molecules, allowing them to replace each other.
Salts are insoluble in hexane (C6H14) because the ion-induced dipole interactions between the ion and the nonpolar hexane are relatively weak and cannot compensate for the strong ionic attractions.
Because the weak dipole–induced dipole interactions between oil and water molecules cannot substitute the strong H bonds between water molecules or the vast dispersion forces inside the oil, oil is insoluble in water.
Because dispersion forces in one can replace dispersion forces in the other, oil is soluble in hexane.
To investigate these concepts further, consider the solubilities of a sequence of alcohols in water and hexane (CH3CH2CH2CH2CH2CH3), two solvents with extremely distinct intermolecular interactions; polar water molecules display H bonds, whereas nonpolar hexane molecules exhibit dispersion forces.
Alcohols are chemical molecules with dual polarity, consisting of a polar hydroxyl (OH) group linked to a nonpolar hydrocarbon group:
Colloids are classified by the physical states of the dispersed and dispersing substances and involve many combinations of gas, liquid, and/or solid.
Colloids have extremely large surface areas, scatter incoming light (Tyndall effect), and exhibit random (Brownian) motion.
Membranes having holes of 0.0001–0.01 m may remove undesirable ions from water. Remember that osmotic pressure is created when various concentrations of liquids are separated by a semipermeable barrier.
A pressure larger than the osmotic pressure is given to the highly concentrated solution in reverse osmosis to push water back through the membrane and filter out ions. Toxic heavy metal ions such as Pb2+, Cd2+, and Hg2+ are eliminated in this manner in houses.
On a big scale, reverse osmosis is used for desalination, which can convert saltwater (40,000 ppm ion concentration) to potable water (400 ppm ion concentration) (Figure B13.3). Over 18,000 desalination units exist globally, supplying water to 300 million people.
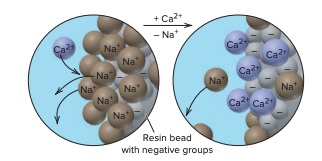
The hard-water cations displace the Na+ ions and bind to the anionic groups. When all resin sites are occupied, the resin is regenerated with a concentrated Na+ solution that exchanges Na+ ions for bound Ca2+ and Mg2+.
The majority of the water used for human use originates from lakes, rivers, reservoirs, or groundwater.
This vital resource may contain soluble hazardous chemical compounds as well as high quantities of NO3 and Fe3+, colloidal clay and microorganisms, and suspended detritus.
Water is treated to eliminate dissolved, dispersed, and suspended particles.