Year 8 Science Term 2 Test
Plants
Photosynthesis
Biotic factors are living components of an ecosystem, such as plants and animals, while abiotic factors are non-living components, such as temperature and water. Trophic levels refer to the levels of feeding relationships in an ecosystem, with producers at the bottom and top predators at the top. Feeding relationships describe how organisms obtain energy and nutrients, with herbivores eating plants, carnivores eating other animals, and omnivores eating both. These factors and relationships are important in understanding the dynamics of ecosystems and how environmental changes impact them.
Photosynthesis is the process carried out by plants to produce food. The chemical equation for photosynthesis is: Carbon Dioxide (CO2) + Water (H2O) → [Sunlight and Chlorophyll]→ Glucose (C6H12O6) + Oxygen (O2)
Photosynthesis occurs on the leaves of the plant.
Leaves, Roots, and Stems
Roots
Roots’ Purpose: Roots anchor the plant and help the plant to obtain water and minerals from the soil.
Roots’ Structure: The main root; lateral roots, which spring off the main root; the root hairs, which maximise surface area to uptake more water and minerals. All of the roots have conducting tissue.
Stem
Stem’s Purpose: The stem holds up the plant and is used like a conveyor belt to transfer nutrients around the plant. Many organs are attached to the stem
Stem’s Structure: Xylem, which transfers water and minerals upwards, has cell walls made of lignin, which makes the walls strong; the Phloem, which transfers sugars around the plant (translocation) in any direction, has cell walls made up of sieve cells and companion cells. Together, the Xylem and Phloem form the conducting tissue.
Leaves
Leaves’ Purpose: The leaves create the plant’s food with photosynthesis and are also needed for transpiration.
Leaves Structure: The leaves have a very detailed structure:
The stomata are openings at the bottom of the plant that can release water and allow for the exchange of gases, they are flanked by two guard cells.
Guard cells swell when the plant is hydrated to open stomata allowing for transpiration and gas exchange, they also close the stomata to prevent water loss when nessecary.
The Spongy Mesophyll has tunnels for the exchange of gasses.
The Palisade Mesophyll is where photosynthesis occurs.
The upper and lower epidermis produce the cuticle.
The thin, waxy cuticle comprises pavement cells that protect the leaf from foreign substances.
Ecology
Biotic and Abiotic factors
Biotic factors are classified as living or non-living.
Abiotic factors are classified as non-living.
For example, using the Sydney Harbour ecosystem.
| Biotic | Abiotic |
|---|---|
| Marine Animals | Temperature |
| Birds | Soil type |
| Humans | Salinity levels |
| Trees | Rainfall patterns |
| Bacteria | Amount of light |
Trophic levels and feeding relationships
Feeding Roles
Producers: Organisms that use photosynthesis to make their own food from the sun’s energy.
Consumers: An organism that relies on other organisms for its food.
Decomposers: An organism that breaks down organic matter and releases the energy back into the ecosystem.
Predator: An animal that hunts for its food.
Prey: An animal hunted for food.
Herbivore: An animal that only eats plants.
Carnivore: An animal that only eats meat.
Omnivore: An animal that eats both meat and plants.
Detritivore: An organism that feeds on dead organic material.
Niche: The role and position of a species in an ecosystem.
Feeding relationships
Parasitic relationship: One organism hinders while the other thrives.
Symbiotic relationship: Both organisms benefit.
Predator-prey relationship: One is the “hunter”, and one is the “hunted”
Ways to Describe an Ecosystem
Population: A group of the same species living in the same place simultaneously.
Species: Organisms with similar structures that mate to produce fertile offspring.
Habitats: Where a population lives.
Abundance: The number of organisms of one species within a habitat.
Distribution: The location or arrangement of the species within a habitat.
Community: All of the species living in one habitat interact with abiotic factors.
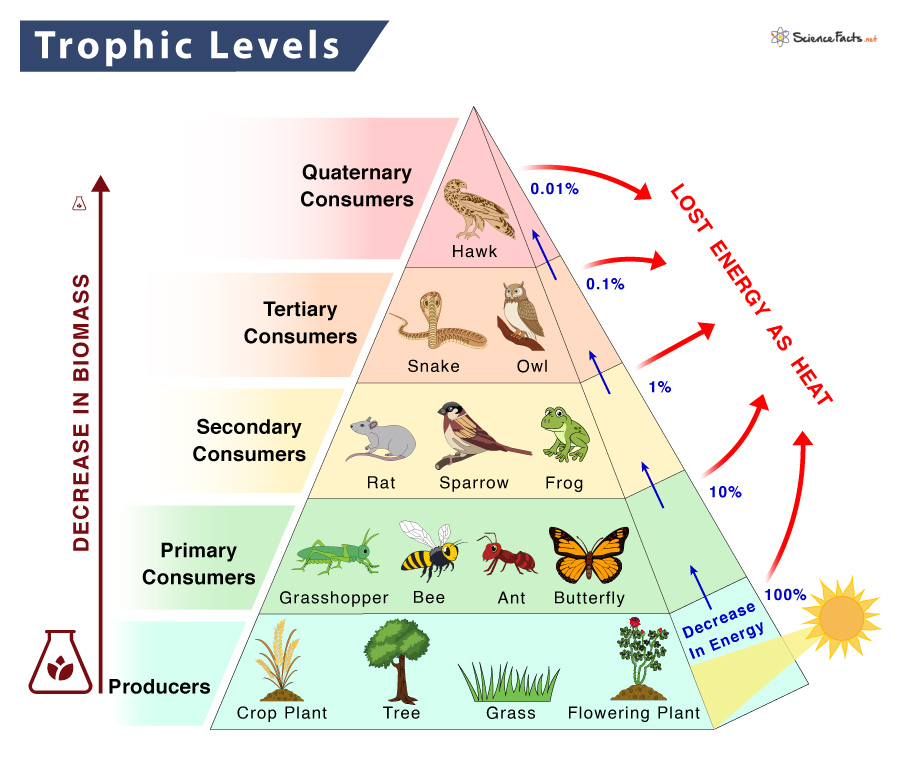
Trophic levels
The trophic levels represent the amount of energy that an animal gains from eating prey, depending on how many animals the prey ate and so on. The levels are as follows: producers, primary consumers, secondary consumers, tertiary consumers, and quaternary consumers. The animal at the top of the food chain is the ‘Apex Predator’.
Biomass
Biomass is the dry mass of the organisms at that trophic level. For example, the producer must have a high amount of biomass to support the apex predator in a balanced ecosystem. It is difficult to predict the effect of an increase or decrease of a small amount of biomass on a large amount of biomass.
Food Chains/Webs
Food chains and webs show the direction of energy flow in an ecosystem. In a food chain, each organism only has one role, but in a food web, each organism can have many roles. When creating food webs, we follow the trophic level system and use arrows to show which direction the energy flows.
Chemistry
The Periodic table
The periodic table is a grid that organises the elements by increasing atomic numbers. The table is split into groups (vertical columns), periods (horizontal rows), and families, like noble gases. Each table element has a name, a symbol, an atomic number (amount of protons) and an atomic weight (protons + electrons).
Metals and Non-metals
Metals
Metals are elements that conduct heat and electricity, are generally solid at room temperature, have a high melting point, are Malleable (can beat into different shapes), are ductile (can turn into wire), and are shiny when polished.
Metalloids
Metalloids share properties with metals and non-metals.
Non-metals
Non-metals are elements that don’t conduct heat and electricity well, melt and turn into gasses easily, are brittle, look dull, glassy and often coloured.
Elements, Compounds, Atoms, and Molecules
Elements
Elements are a sample of matter containing only 1 type of atom. They cannot be broken down into other substances. They all have specific properties
Atom
A sample of matter that uniquely defines a chemical element, is the building blocks of matter.
Compounds
Compounds contain two or more types of atoms that are chemically bonded in a fixed ratio.
Molecules
Molecules contain two or more atoms that are chemically bonded in a fixed ratio
Lattices
Atoms bonded together in continuous frameworks. For example, a million carbon atoms all connected to each other.
Notes
All compounds are molecules, but not all molecules are compounds. The properties of a compound can be vastly different from the atoms that form it.
Locations of sub-atomic particles
An atom comprises three types of particles: protons, neutrons and electrons. Atoms are mostly comprised of empty space.
Protons
Protons have a mass of 1. They are located in the nucleus, the centre of the atom. Protons have a positive electrical charge.
Neutrons
Neutrons have a mass of 1. They are located in the nucleus. They have a neutral electrical charge.
Electrons
Electrons have a mass of 1/1840. They zoom around the nucleus in constantly changing paths called orbits, which are very far away from the nucleus. Electrons have a negative electrical charge.
Notes
The electrical attraction between the nucleus and the electrons keeps them orbiting the nucleus. A neutral atom (any in the periodic table) has equal numbers of protons an electrons. The nucleus has an overall positive charge.
Word Equations
Chemical equations are a shorthand way of representing chemical reactions. The reactants are the substances that are present before the reaction occurs, while the products are the substances that are formed as a result of the reaction. In a chemical equation, the reactants are written on the left-hand side of the arrow, while the products are written on the right-hand side. The arrow represents the direction of the reaction, from the reactants to the products. Sometimes, we write what process we do to the reactants above or below the arrows. Eg ‘heat’ or ‘sunlight’.