Geogirl + other lecture videos
Modal vs Norm Mineralogy, Major vs Trace Elements, & Indices- Igneous Petrology #7
Major elements in Igneous Rocks
Igneous rocks that come from a common source (parent magma) can have various compositions due to differentiation
Compatible elements: preferentially incorporated into the crystals from cooling melt
Incompatible elements: Stays in melt (for longer)
The first minerals to crystalize from magic melts are silica poor -→ differentiation causes residual melts (left over melts) to increase in silica content
AKA: silica behaves as an incompatible element
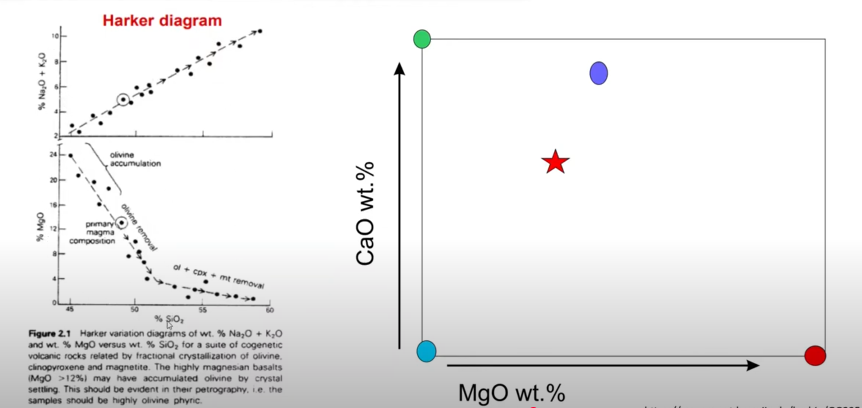
→ We can measure the concentration of other elements against SIO2% to show their concentration change through increased differentiation (Harker diagrams)
Ferromagnesian and calcic plagioclase minerals (CaO, FeO. MgO, TiO2) tend to decrease with increasing SiO2
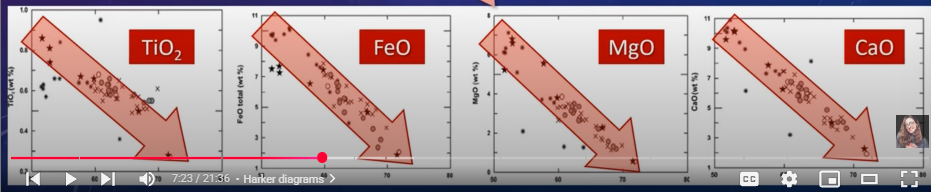
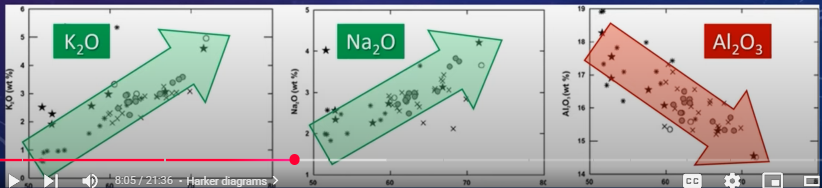
Al2O3 may also decrease, but not as drastically
Alkalis (K20 and Na2O) increase with increasing silica
Major Element Indices
In addition to Harker diagrams, other chemical variation diagrams can help us reconstruct the differentiation history of magmas.
5 major element indices of differentiation:
Alkali-Lime Index
Iron-Enrichment Index
Aluminum Saturation Index
Alkalinity Index
Feldspathoid Silica-Saturation Index
Alkali-Lime Index
Based on relative abundance of CaO, Na2O, and K2O
If CaO is more abundant, the first feldspar to crystallize will be anorthite (Ca-Spar)
If alkalis are more abundant, the first feldspars will be albite (Na-spar) and orthoclase (K-spar)
Divided into 4 categories of relative Ca and K/Na content:
-Alkalic (<51% SiO2 where CaO= Na2O+K2O)
-Alkalic-calcic (51-56% SiO2)
-Calc-alkalic (56-61% SiO2)
-Calcic (>61% SiO2)
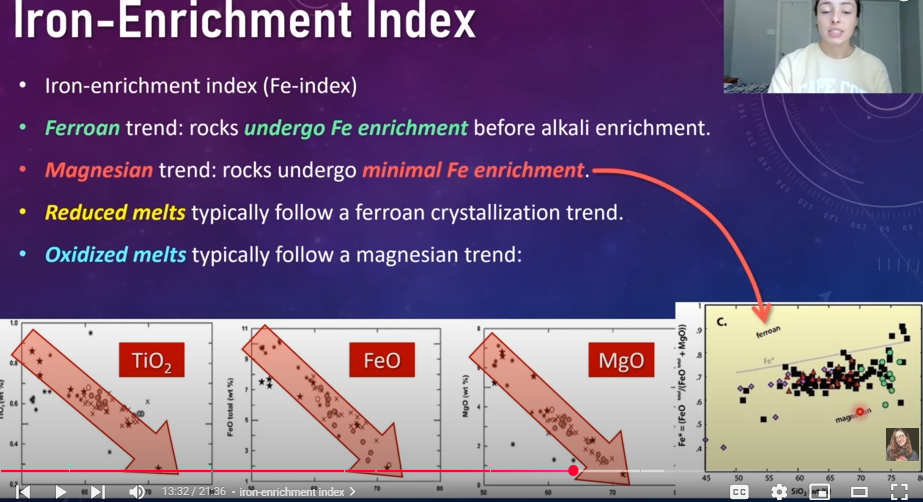
Iron-Enrichment Index
Ferroan trend: rocks undergo Fe enrichment before alkali enrichment
Magnesian trend: rocks undergo minimal Fe enrichment
Reduced melts typically follow a ferroan crystallization trend
Oxidezied melts typically follow magnesian trend
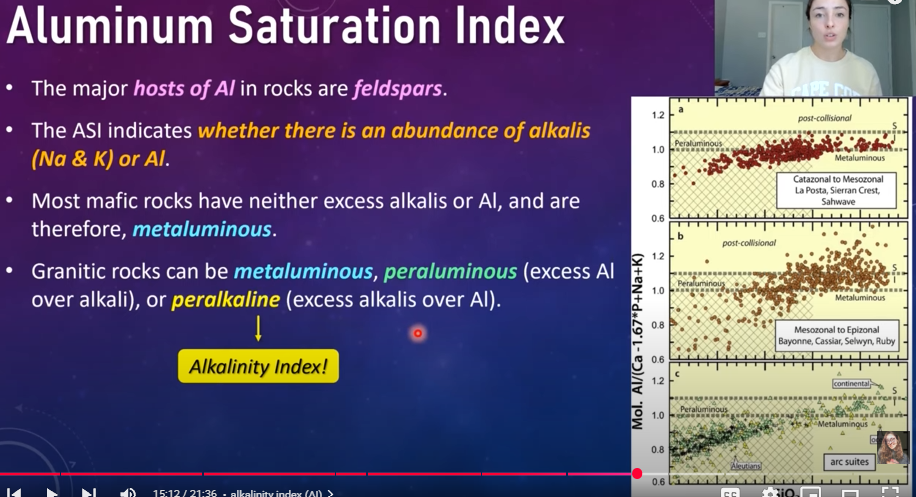
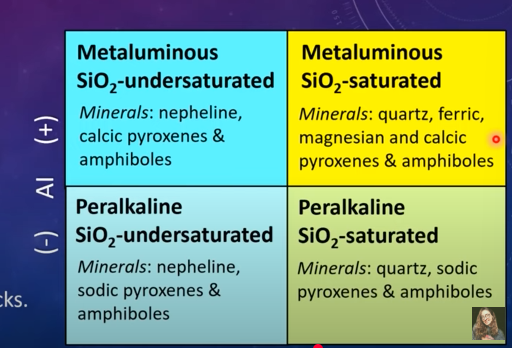
Aluminum Saturation Index
The major hosts of Al in rocks are feldspars
The ASI indicates whether there is an abundance of alkalis (Na and K) or Al
Most mafic rocks have neith excess alkalis or Al, and are therefore, metaluminous
Granitic rocks can be metaluminous, peraluminous (excess Al over alkali), or peralkaline (excess alkalis over Al)
Alkalinity Index (Al)
AL- [Al- alkalis (Na+K)]
Metaluminous: Positve Al
Peralkine: Negative Al
Feldspathoid-Silica-Saturation Index (FSSI)
-Qz excess over leucite and nephline
Positive for quarts saturated rocks
Negative for silica undersaturated rocks
Use of Trace Elements
Trace elements can be extremely useful for identifying the conditions and processes that led to the formation of igneous rocks
Why?→ Trace elements (<0.1%) show such drastic concentration changes in rocks depending on conditions (compared to major elements)
Common trace elements:
Transition metals: Sc, Ti, V, Cr, Mn, Co , Ni, Cu, Zn
Rare Earth Elements (REES): La, Ce, Nd, Sm , Eu….
Others: Cs Rb, Ba, Sr, Y, Zr, Hf, Nb
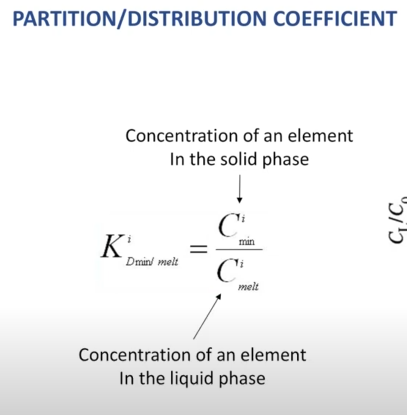
Trace elements are partitioned between the minerals and melt: Partition coefficient (D)= Ci min/Cimelt (where c is the concentration of the trace element, I, in other mineral (min), over the melt)
Compatible elements concentration in the Mineral (high D, or partition coefficient value)
Incompatible elements concentrate in the melt (Low D, or partition coefficient value)
Phase Diagrams: Minerals Melt at Different Temperatures- Igneous Petrology #5 | GEO GIRL
Magma Viscosity, Volatile Content, Partial Melting, & Mixing- Igneous Petrology #6 | GEO GIRL
Magma Differentiation
Partial melting
-Equilibrium melting; Solid complete melts without melted fraction leaving the system (final liquid= same composition as original solid)
-Fractional melting: partial melt is removed incrementally (final liquid= differentiated)
All partial melts are more felsic that their source rock
Crystallization:
Equilibrium crystallization: crystals don’t leave the system (final sold= same composition as original melt)
Fractional crystallization: crystals are removed (by gravitational settling) causing changes to the remaining liquid’s composition (final solid= differentiated)
Order?→ Bowen!
Assimilation: host rock torn off and incorporated into rising magma
Magma mixing: Magma chambers my mix if they come in contact at depth, and the resulting magma will rise to the surface (or cool near surface)
Magma differentiation can also occur if grains crystallize and settle to the bottom of the chamber, as we saw from fractional crystallization.
Magma Chamber Processes: Fractional Crystallization
Groups: same number of electrons in their outer shell
Periods: same number of shells
Large Ion Lithophile Elements (LILE)
Small charge/ionic radius
High Field Strength Elements (HFSE)
Elements will a small ioinc radius (Z/r) and high charge (and high associated electric field)
Includes all trivalent and tetravelent ions
Goldscmits classification
Igneous Rock Textures & Classification Based On Grain Size & Shape- Igneous Petrology #2 | GEO GIRL
How/why does crystallization begin?
Reactions (such as crystallization must have a G<0 to occur spontaneously
What is G? → Gibbs free energy (calculated by Enthalpy (H) and Entropy (S)
What is enthalpy? → The heat stored in the bonds of a substance
Because bond strengths vary, heat can either be released or consumed due to a chemical reaction
When change in enthalpy (Delta H) is negative (heat was released), the reaction is exothermic and can occur spontaneously
But there is another component to this equation!
What us S? → Entropy! → what is entropy? → measure of disorder/randomness
Example: elements in gases have higher entropies
If S increases during a reaction, the reaction will have an easier time proceeding
However, if it decreases the reaction may not proceed.
Example: If deltaS is so low that G becomes positive, even if delta H is negative, the reaction will not occur spontaneously
Equation:

So, if H decrease (negative) and S increases (+), G will be <0 (negative, and proceed spontaneously
So is crystallization a spontaneous process?
It involves building bonds (releasing hear, AKA: Negative H)= exothermic and spontaneous.
But what about S? Entropy decreases during crystallization because the atoms are becoming more orederd, but this is typically outweighed by the H decrease
However, even when crystallization reaction has negative G value, there may be an energy barrier to overcome
Igneous Petrology Series: Lesson 2 - Element compatibility & partition coefficients
Bulk distribution Coeffiction
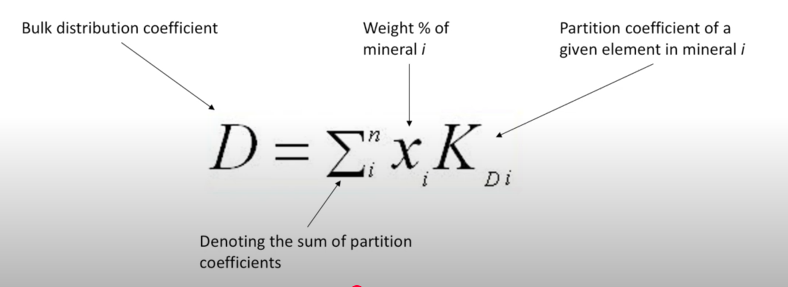
Element compatibility:
In geological systems, element compatibility categories elements based on their behavior during processes such as cstyallisation and melting. It essentially represents how readily a trace elemt sil substitute for a major element in a mineral
A trace element can sub for a major element when its ionic radius and valence state are similar to that of a major elemt. For ex. Eu2+ is similar to Ca2+ and so can replace that ca in plagioclase felspar
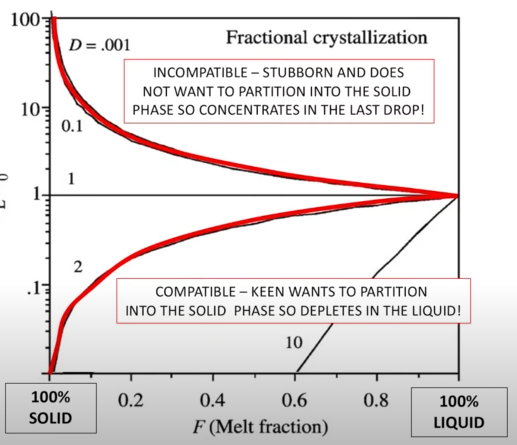
An incompatible element generally refers to an element with D value below 1. During melting, an incompatbile leemt will be the first to partition into melt phase, and last to partition into sold during crystallization
A compatible eleemt refers to cement with D values over 1. Higher amounts o fmeltin will be require to libearte a compatible ement fro the solid phase into the melt, and during crystallization nis is one of the fisr elements to become a solid
This behavior generally means that crust is relatively enriched in incompatible elemtns realtve to the mantle and vice versa for compatible elements
Igneous Petrology Series: Lesson 3 - Gibbs Phase Rule
Identifies the degrees of freedom in a system using the number of components and the number of phases
In more simple words, it tells use how many intensive variables needed to define a system
Number of components (denoted as C) = number of components existing in the system
Number of phaseses (denoted as P)= number of phases existing in the system
Parameters:
Intensive
Variables that don’t scale with a system
AKA parameters that are not proportional to the system
Good examples are temperature and pressure. Just because a system is bigger to smaller, doesn’t mean the temperature has to be!
Extensive:
Variables that don’t scale with a system
AKA parameters that are proportional to the system
Good examples are volume and mass. The bigger the system is, the bigger these values are
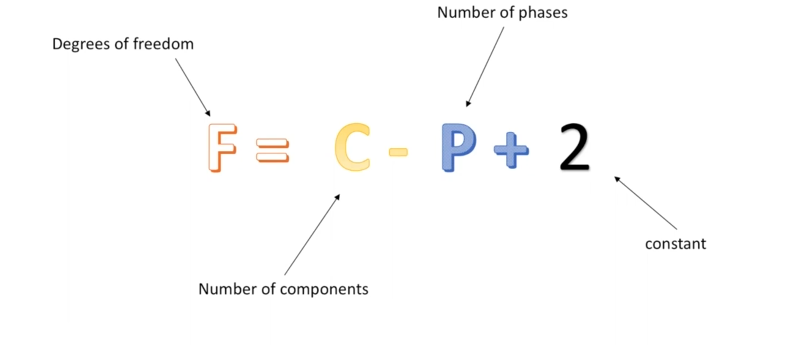
Geochemical Data Series: Lesson 1 - Major, minor, and trace elements
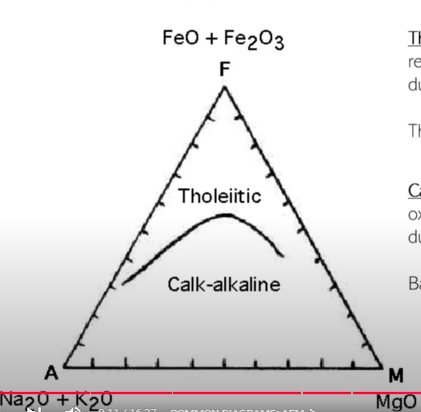
Common Diagrams: AFM
Toleiitc rocks are sub-alkaline rocks that crystallize from reduced magmas. In this trend, Fe generally initially increases due to the precipitation of Mg-rich minerals
Tholeiitic basalt → ferrobasalt → tholeiitic andesite → dacite
Calc-alkaline rocks are subalkaline rocks that crystalized from oxidized magmas. In this trend, Fe gernally initially decreases due to the precipitation of Fe-rich minerals
Basalt → andesite → dacite → rhyolite
Harker and Fenner Diagrams
Geochemical Data Series: Lesson 2 - Rare earth elements
Rare Earth Elements → A group of (typically) trivalent metals that have similar physical and chemical properties.
Lanthanides → A group of 15 REEs that have atomic numbers from… each have a 5d valence electron
LREE/MREE/HREE→ Light, middle, and heavy rare earth elements
Lanthanide contraction → phenomenon where the ionic radii of the lanthanides decreases with increasing atomic number
Odo-Harkin’s rule → An element with an even atomic number is more abundant that adjacent elements with odd atomic numbers
In geology, we use REE’S to evaluate processes like mantle melting, fractional crystallization, and crustal contamination
All are generally trivalent and considered incomaptbile… but compatibility is a spectrum, and slight difference in partition coefficient can lead tp patterns we interpret in spider diagrams
Why do we normalize?
Bc of the Oddo-Harkin’s effect, you see zig zag pattern.
To counteract, we normalize REE’s (i.e divide our sample REE concentration with REE concentrations of a well-constrianed reservoir).
The most common normalization values include: chrondrites, primitive mantle, upper lower crust, N-MORB.
In reduced environments Eu3+ can become Eu 2+, which is similar in size and charge to Ca2+, and this becomes compatible in Plagioclase feldspar.
Negative anomalies suggest Eu2+ has been removed from our samples either by plagioclase remaining in the source (residuum) or that some plag has crystallized.
Positive anomalies suggest our rock has some accumulation of primary plag

 Knowt
Knowt