Tyrosine Kinases
Tyrosine kinases are a specialised kinase subset. They are particularly important in growth/cell division and metabolism. Examples include c-Src (the first proto-oncogene) and the intrinsic tyrosine kinase found in the insulin receptor b subunit cytosolic domain.
Insulin receptor:
a2b2 polypeptide
stabilised by disulphide bonds
insulin binds a2 subunits
signal transmitted to b2 → tyrosine kinase activation
signal has crossed membrane
The tyrosine kinase phosphorylates specific Tyr residues in the receptor. This is known as auto-phosphorylation as the receptor is phosphorylating itself. The phosphorylated Tyr residues recruit signalling molecules eg IRS-1 to the receptor which are then also phosphorylated, forming a signalling complex.
How does ligand binding activate a receptor?
dimerisation of receptors (insulin receptor already a heterodimer) (see pic below for visualisation) → movement of activation loop
ligand induced activation of tyrosine kinase - this is explained further down
autophosphorylation on specific tyrosines in the cytosolic tail of the kinase
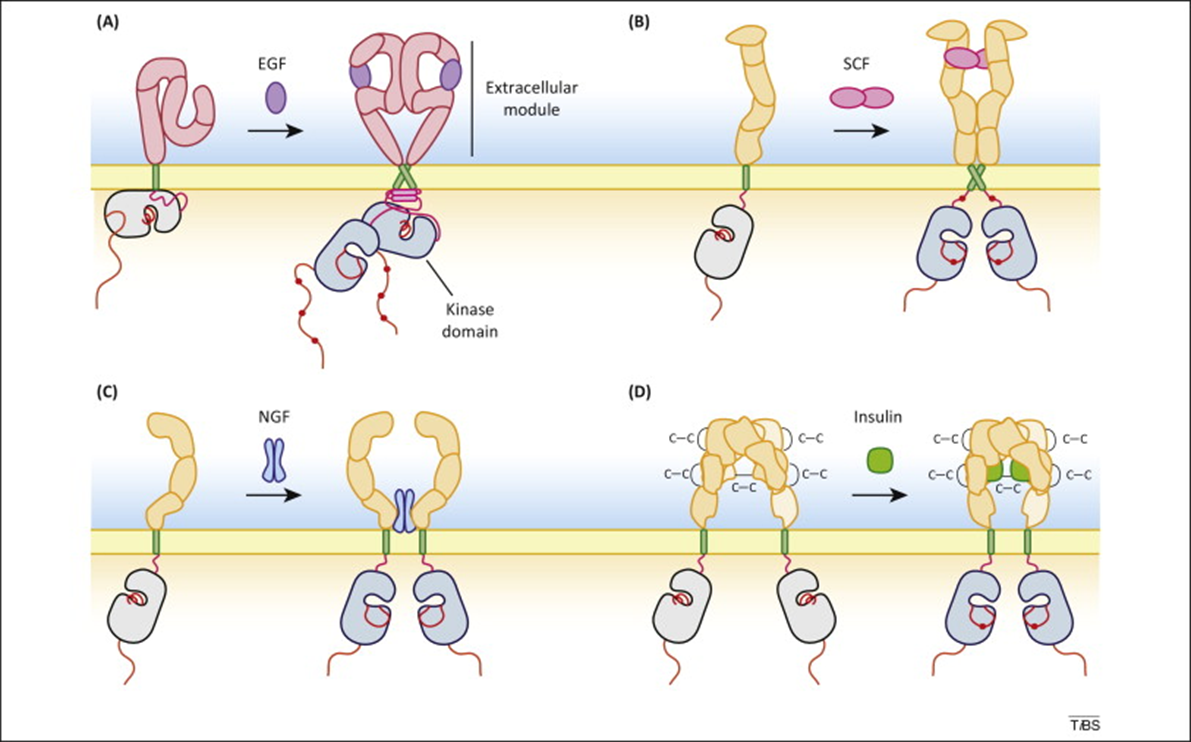
The picture above shows dimerisation of receptors caused by the extracellular binding of their ligands eg epidermal growth factor to the EGF receptor, nerve growth factor to the NGF receptor or scatter factor/hepatocyte growth factor to the SCF receptor.
How is intrinsic tyrosine kinase activity of the receptor activated? When the ligand binds, the resulting binding ‘signal’ is transmitted across the membrane. This results in a conformational change activating the intrinsic tyrosine kinase of the receptor. The receptor is autophosphorylated.
The insulin receptor is already a heterodimer. When insulin binds, the alpha subunits ‘intertwine’ which brings the beta subunits closer together. This makes autophosphorylation easier. This has been observed by incorporating the receptor into an artificial system called a nanodisc, and using high resolution cryo-electron microscopy to look at structural changes when insulin is added.
Trans autophosphorylation: activation of tyrosine kinase domain in receptor promotes phosphorylation of specific tyrosines in the receptor. The dimeric receptor chain A phosphorylates tyrosine in chain B and vice versa. Phosphorylated tyrosine residues act as magnets for the arrival of key signalling molecules - they act as docking sites. SH2 domains are an example protein domain in many other proteins which bind phospho-tyrosine residues.
Autophosphorylation of specific tyrosines occurs to attract SH2-domain containing proteins to the receptor which are then activated, sending a signal. The SH2 domain is encoded by a single exon. Different receptors attract different proteins.
Although all SH2 domains bind phosphotyrosine and contain a ‘pocket’ P-Tyr fits into, they don’t all bind ALL P-Tyr residues. Specificity in P-Tyr residues is created by the surrounding sequence in the receptor polypeptide - each SH2 domain has a different specificity pocket eg EGF receptors recruit a subset of SH2 domain containing proteins eg PLC-gamma or Ras-GAP. These proteins bind specific tyrosine residues in the receptor. This is sometimes thought of as a two-pronged plug concept.
Different receptors assemble different protein complexes depending on effectors recruited. The effectors recruited is due to what proteins are expressed in a particular cell type. This means a receptor can have different functions in different cell types due to having different effectors downstream in the pathway.
Leptin pathway: Leptin is a hormone produced by fat cells. Leptin binds its receptor which homodimerises and autophosphorylates. The receptors associate with JAK2 and transcription factor STAT3 binds. STAT3 is phosphorylated and dimerises. The dimer enters the nucleus and binds genes allowing them to be transcribed. Leptin is used in mouse models of obesity.
PLC-γ activation:
sig molecule binds GPCR
GPCR alpha subunit activated
activates phospholipase C
phospholipase C cleaves inositol phospholipid → IP3 and DAG
IP3 binds receptor on ER calcium channel → calcium ion release
DAG and calcium bind phosphokinase C activating it
Activation of PLC gamma by tyrosine kinase receptors:
PLC gamma recruited via SH2 domain to receptor therefore PLC gamma close to TK domain of receptor and is phosphorylated by tyrosine. Now active and near substrate.
Activation of the MAPK cascade:
Background: Ras is a G protein. Ras-GTP activates a signalling pathway whereas Ras-GDP is its inactive form. Ras-GTP:Ras-GDP is controlled by the relative activity of Ras-GAP and SOS proteins. Recruiting these proteins to receptors via SH2 domains is therefore a key control step.
Pathway: Tyrosine kinase receptor phosphorylate Shc which recruits Grb2, SOS and Ras. Ras activates Raf1 kinase which phosphorylates MEK 1/2. MEK 1 / 2. ERK 1 / 2 activates MAPK (mitogen activated protein kinase). However, the tyrosine kinase receptor may phosphorylate Ras-GAP which does not phosphorylate Ras, stopping the pathway. The pathway is also stopped by the production of ERK 1/2, acting as a negative feedback loop by inhibiting Raf1 and SOS activity.
Raf aka MAPKKK, MEK aka MAPKK, ERK aka MAPK.
Purpose: this pathway is involved in cell growth. Tyrosine kinases activate or inhibit growth and cell division depending on their Ras input.
Kinases often function in a pathway to create massive, specific amplification of signals.