Biology Flashcard (Science Bowl)
Biology
Subject: Neurology
Embryology
Embryology:
Neuroanatomy
Cerebral Cortex:
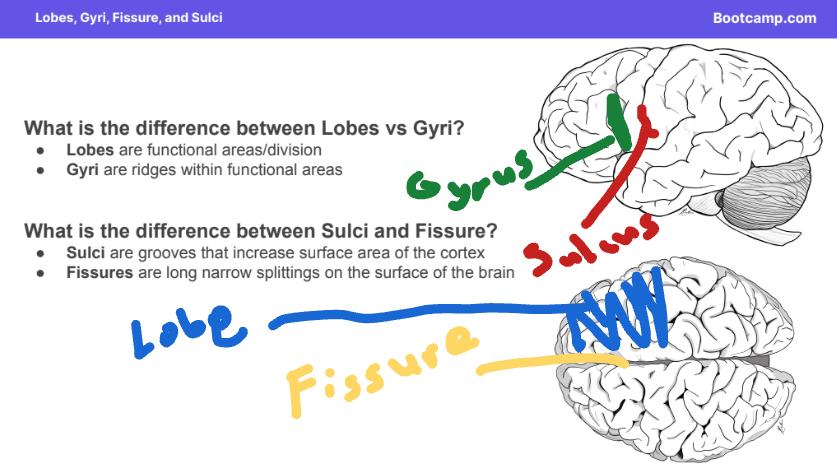
Lobes: Larger area of functional areas where gyri group together.
Gyri: Ridges found in brain lobes (Singular: Gyrus)
Sulci: Grooves that increase the brain’s surface area (Singular: Sulcus).
Fissures: Long splitting of the brain
Sulci folds the brain to increase its surface area, allowing it to fit inside the skull.
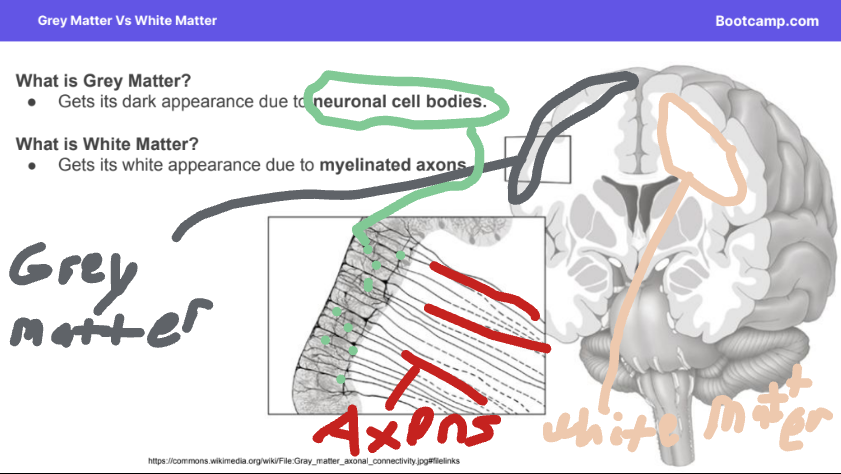
The cerebral cortex gains its function by the types of cells that make it up. There are two types of parts grey matter and white matter.
Grey Matter: Made up of neuronal cell bodies causing it to have a dark appearance.
White Matter: Made up of myelinated axons giving it a white appearance.
Grey matter is located at the top of the cerebral cortex. There are a few grey matter spots on the inside of the cerebral cortex.
White matter is located on the inside of the cerebral cortex.
The neuronal cell bodies are made up of dendrites that communicate and send information down the axons.
Myelinated axons have a high concentration of lipids. This increases the conduction of the axons making communication between cells faster.
Subject: Immunology
T Cells
T Cell Activation: The process of turning T cells into effector T cells from Naive T cells. Antigen Receptor: The protein in which recognition of an antigen occurs. Antigen Presentation: The display of foreign fragments to activate T cells. | Types of T Cells
Activation
T Cell Activation Signals
|
Subject: Reproduction
Animal Development
Development: The changes an organism undergoes as it grows older. Fertilization: The formation of a diploid zygote from a haploid egg and sperm. Acrosome: A special vesicle on the top of the sperm head that holds hydrolytic enzymes. Polyspermy: The entry of multiple sperm nuclei. Cortical Reaction: The reaction is believed to be caused by increased Ca2+, which provides long-term prevention of polyspermy by fusing cortical granules. Cleavage: a series of cell divisions during development. Blastomeres: Smaller cells that are the result of cleavage. Blastula: A hollow ball of cells from the first five to seven cleavages. Blastocoel: The fluid-filled cavity surrounded by the blastula. Morphogenesis: The process in which an animal's body develops over time during embryonic development. Gastrula: Two or three-layered embryos created through gastrulation. Diploblasts: Embryo with two layers. Triploblasts: Embryo with three layers. Germ Layers: The cell layers produced in gastrulation. Ectoderm: The layer that forms on the outer gastrula. Endoderm: The layer that forms on the inner gastrula. Mesoderm: The layer that forms between the ectoderm and endoderm in triploblasts. Organogenesis: The development of the rudimentary organs in the embryo. Neurulation: The process in which the neural plate forms the neural tube by folding in on itself. Induction: A process in which a group of cells or tissue influences the development of other groups through close interactions. Hox Genes: Master regulatory genes that control the positional identity of an embryo. | Steps of Embryonic Development I Fertilization An acrosomal reaction occurs when the sperm makes contact with the egg. The acrosome discharges hydrolytic enzymes. The enzymes digest the egg jelly coat allowing for the acrosomal process and fusion to begin. This begins fertilization, a process that speeds up metabolic reactions to bring embryonic development. The cell is now activated. II Cleavage During cleavage, phases G1 and G2 are essentially skipped. Only phases S and M take place. The first five to seven cleavages result in a blastula. Holoblastic Cleavage: In this cleavage, cleavage passes through the entire egg (found in echinoderms, mammals, and annelids) Meroblastic Cleavage: In this cleavage, the volume of the yolk is so great that a cleavage furrow cannot pass through it. The region lacking yolk goes through cleavage as a result. III Gastrulation During this stage, a set of cells move into the blastocoel. This then leads to cell layers and a digestive tube to be formed. Ectoderm = nervous system, skin, and pituitary gland Mesoderm = skeletal and muscular systems, circulatory and lymphatic systems. Endoderm = digestive tract and organs, thymus, thyroid, and parathyroid. IV Organogenesis During this stage, the regions of the germ layers develop into the rudimentary organs. Neurulation will be an example of how different cells are fated for certain roles in development (Neurulation is the first step in organogenesis). Neurulation begins as cells in the dorsal mesoderm form the notochord. Signaling molecules are released from these cells to create a neural plate in the ectoderm (This is an example of induction). The neural plate folds inwards and forms the neural groove which eventually forms the neural tube. This tube will give rise to the neural tube. The neural tube is the foundation for the brain and the spinal cord |
Human Development
Human Eggs: Small eggs with little food reserve. Oviduct: The tube where fertilization occurs, between the ovaries and uterus. Cleavage: The division of the fertilized egg into multiple cells. Blastocyst: Hollow ball of cells formed after cleavage. Inner Cell Mass: The group of cells within the blastocyst that will develop into the embryo. Trophoblast: Outer layer of the blastocyst responsible for implantation. Endometrium: Lining of the uterus that the blastocyst embeds into. Epiblast: Inner layer of cells in the inner cell mass that will form the embryo. Hypoblast: Outer layer of cells in the inner cell mass that supports the development. Extraembryonic Membranes: Membranes that surround and support the embryo. Placenta: Organ that allows nutrient, gas, and waste exchange between mother and embryo. | Fertilization occurs in the oviduct where the sperm and egg fuse. Development begins after fertilization. After fertilization, the embryo begins its journey to the uterus, completing development as it travels. Cleavage divides the embryo into over 100 cells, forming a blastocyst with an inner cell mass. The blastocyst travels to the uterus and forms a cluster of cells at one end, known as the inner cell mass. The inner cell mass forms the actual embryo, with the surrounding trophoblast aiding in implantation. The trophoblast secretes enzymes to break down the endometrium and facilitates implantation into the uterus. Implantation occurs when the blastocyst embeds into the endometrium, beginning the next stage of development. After implantation, the epiblast forms the ectoderm, mesoderm, and endoderm, which are the three germ layers. The hypoblast contributes to the formation of extraembryonic tissues, such as membranes, to protect and support the embryo. These extraembryonic membranes surround the embryo and support its growth, also forming structures like the placenta. The placenta forms from the epiblast, trophoblast, and endometrial tissue, facilitating nutrient exchange and waste removal. |
Subject: Genetics
Basic Genetics
Heredity: The passing of traits from parents to their offspring, with variations in traits caused by genetic factors. Allele: Different versions of a gene. Homozygous: Having two identical copies of a gene. Heterozygous: Having two different copies of a gene. Genotype: The genetic composition of an individual that creates all of their traits. Chromosomes: The bundle form of DNA that is used to pass down traits. Polygenic Trait: A trait that is expressed due to the presence of multiple genes. Mendelian Trait: A single trait that is expressed by a single gene. Pleiotropic Gene: A single gene that controls how multiple traits are going to be expressed. | Heredity Gregor Mendel Long ago, people had already realized that offspring usually had the same traits as their parents. However, no one knew exactly how or why this occurred. Gregor Mendel is considered the father of heredity and classical genetics. He experimented on pea plants in his garden and discovered the framework of how traits get passed from one generation to the next. Classical Genetics This part of genetics includes the ideas Mendel had about how traits are passed down. In your chromosomes, many genes express your traits. All cells other than reproductive cells (Gametes) are diploid and receive two copies of each chromosome, one from each parent. The chromosomes are not identical, allowing for variation of your traits. |
Mandel’s Laws
Law of Dominance: The law that states that dominant alleles in heterozygous genotypes will mask the expression of the recessive allele, resulting in the dominant phenotype being expressed. Law of Segregation: The law that states individuals possess two alleles and a parent only passes one of his/her alleles to their offspring. Heredity Probabilities Homozygous Dominant (AA) with Homozygous Recessive (aa) = 100% Dominant, 0% Recessive 2 Heterzygous (Aa) = 75% Dominant, 25% Recessive Heterozygous (Aa) with Homozygous Recessive (aa) = 50% Dominant, 50% Recessive Heterozygous (Aa) with Homozygous Dominant (AA) = 100% Dominant, 0% Recessive | Punnet Squares Punnet squares can be used to determine the probabilities of traits passed down to the offspring of parents. It works by identifying the possible outcomes of a dominant and recessive gene. The pairs of genes are crossed on a table showing the possibilities. You can then check the possibility of an offspring receiving either a dominant or recessive gene (Remember: Dominant genes will always be expressed over recessive genes). |
Subject: Evolution
Natural Selection
Evolution: The change of a population’s inherited traits over time. Population: A group of organisms in the same species in the same place. Gene Flow: Genes that move through a different population Genetic Drift: The change in genes caused by a random event Natural Selection: A process that favors the traits needed in an environment. | Evolution happens between populations, not individuals. Each member of a population has different traits. Mechanisms that cause changes in the population’s traits can lead to evolution. Different Evolution Mechanisms Gene Flow allows for genes to be transferred from one population to the next. Genes can mutate and create new genes. Genetic drift can change the gene pool of an entire population due to random chance. Natural Selection makes it so that a gene pool that is fit for the environment survives. Evidence for Evolution Shared common ancestry creates many similarities between organisms. These are called homologies. DNA homologies are the biggest evidence for evolution. Different species in different areas still share similar genes due to common ancestors. Another homology is in anatomy. Different species have similar anatomy, suggesting that they are related (e.g., human forearm and dog forearm). Vestigial structures were passed down from an ancestor but have no use now. This is huge evidence for evolution as it shows how an organism develops over time. Developmental Homologies show similarities in the development of related species. This suggests that these species are related and have their developmental processes passed down. Fossil Records Fossil records show the remains of long-lost organisms. They can show how a population's characteristics have changed over time. Biogeography This field of science studies how organisms are spread out around the planet and the relationships of these organisms in different areas. This field of science provides evidence that changes in location act to change a species. |
Phylogeny and the Tree of Life
Taxonomy: The branch of biology that deals with classifications of animals, plants, and microorganisms. Phylogeny: The evolutionary history of a species. Cambrian Explosion: A period of rapid evolution where most of the major phyla appeared in the fossil record (541-530 million years ago). Tree of Life: A diagram that shows the evolutionary relationship between all living things. Miller-Urey Experiment: A chemical experiment that simulated the conditions of Earth that allowed for the formation of biomolecules. Important Phyla
| Tree of Life The truck of the tree represents the unicellular origins of life. The branches of the tree represent the different organisms created by centuries of evolution. Major Divisions of Animalia
All organisms belong to a certain species. These are populations that have sufficient genetic similarities for reproduction and produce viable offspring. Origins of Life The origins of life can be traced 4 billion years ago. The Miller-Urey simulated how Earth was when it had cooled down enough for liquid water to exist. This allowed for nitrogenous bases and amino acids to form spontaneously. The amino acids and nitrogenous bases polymerized to form long chains. The chains, by chance, were enclosed in self-generating membranes creating the first protocells. When nucleic acids became self-replicating, inheritance took hold. History of the Earth Hadean Eon
Archean Eon
Proterozoic Eon
Cambrian Explosion
Phanerozoic Eon
Phylogeny Major Divisions of Animalia
Domains are the most basic categorizations of life. It includes
The next classification kingdoms come from the different types of eukarya. These kingdoms include:
In each kingdom, there are several phyla. This includes: Animal Phyla
Plant Phyla
|
Subject: Cells
Cell Structure and Function
All cells are made up of proteins, lipids, nucleic acids, and carbohydrates (Macromolecules). Phospholipids have polar heads and non-polar fatty acid tails. Cell Compartmentalization Cells are organized into subcellular components Members of the endomembrane system
Rough ER
Smooth ER
|
Cell Division
Mitosis: The division of somatic (body) cells |
Tissues
Connective Tissue: Tissue that supports, protects, and binds other tissues. Has an abundant extracellular matrix. Function of Connective Tissue: Provides structural support, stores energy, transports materials, and connects tissues. Tendons: Connective tissue that connects muscle to bone. Ligaments: Connect bone to bone. Cartilage: Connective tissue that provides flexible support (e.g., nose, ear, joints). Mesenchyme: A loose fluid-like embryonic tissue in which connective tissue originates. Extracellular Matrix: The main component of connective tissue that is located outside the actual connective tissue cells and is made of nonliving materials Ground Substance: A watery unstructured material that fills in the space between cells. Fibers: Molecules that give support and shape to the ground substance Glandular Epithelium: A type of tissue that secretes substances (e.g., hormones, enzymes). Simple Epithelium: A single layer of the epithelial cells that allow diffusion, filtration, or secretion (e.g., lung alveoli, kidney tubules). Stratified Epithelium: Protects underlying structures (e.g., skin, mouth lining). Function: Produces movement through contraction, maintains posture, and generates heat. Skeletal Muscle: Moves bones and allows voluntary movement. Cardiac Muscle: Pumps blood through the heart and circulatory system. Smooth Muscle: Moves substances through the digestive tract and regulates blood flow. Nervous Tissue Function: Sends and receives electrical signals to control body functions. Neurons: Cells that transmit electrical impulses to process and relay information. Glial Cells: Cells that support and protect nerve cells in the brain and nervous system. Central Nervous System (CNS): The part of the nervous system that consists of the brain and spinal cord. Peripheral Nervous System: The part of the nervous system that consists of nerves connecting the body to the CNS. | Connective Tissue Connective tissue is the most abundant tissue type, showing up almost everywhere in your body. Loose connective tissue is very open and is found in ligaments. Dense connective tissue is found in tendons, and is tightly packed together. Types of connective tissue
Fat is a type of connective tissue that provides insulation and fuel storage. The bones, tendons, and cartilage bind, support, and protect your organs and make up your skeleton. Your blood transports nutrients and materials throughout the body. Unique Aspect of Connective Tissue
The main components of the extracellular matrix include the ground substance and a multitude of fibers The ground substance is made of
Fibers come in many types
Types of connective tissue cells Blast cells These types of cells are immature stem cells that are specialized to form the specific ground substance and fibers for their specific connective tissue (For example: Osteoblasts and Chondroblasts). Cyte Cells These cells are mature connective tissue cells after the blast cells have created their ground substance or fiber (For example: Osteocytes and Chondrocytes). They maintain the health of the matrix after its creation. Epithelial Tissue Covers body surfaces, lines cavities, and forms glands. Cells are tightly packed with little extracellular matrix. Found in layers: simple (one layer) and stratified (multiple layers). Stratified epithelium provides protection in high-friction areas (e.g., skin, esophagus). Simple epithelium is used in areas where things need to be absorbed (e.g., kidney, stomach). There are three structures of epithelial cells: cuboidal (cube-shaped), squamous (flat), and columnar (tall and rectangular). Muscle Tissue Responsible for movement. Made of elongated cells (muscle fibers) that contract. There are three types of muscle tissue: skeletal, cardiac, and smooth muscle. Skeletal muscle is voluntary, connected to the muscle, and is used for movement. Cardiac muscle is Involuntary, striated, and found in the heart. It has intercalated discs for coordination. Smooth muscle is involuntary, non-striated, found in the walls of organs and blood vessels. Nervous Tissue Composed of neurons and supporting cells. Found in the brain, spinal cord, and nerves. Neurons are the main signaling cells. They contain dendrites that receive signals and axons that send signals. Glial cells (Neuroglia) are nervous system immune cells that support, nourish, and protect neurons. |
Subject: Organ Systems
The Cardiovascular System
The Nervous System
Neuron: The basic subunit of the nervous system that controls | Basic Subunit of the Nervous System (Neuron) |
The Integumentary System
Integumentary System: The body's outer layer that keeps boundaries and regulates the body’s homeostasis. Functions and the How of the Integumentary System: Protects Deeper Tissues from: Mechanical Damage (Bumps) - The physical barrier contains keratin, which toughens cells; fat cells to cushion blows; and both pressure and pain receptors, which alert the nervous system to possible damage. Chemical Damage (Acids and Bases) - Has relatively impermeable keratinized cells; contains pain receptors, which alert nervous cells to possible damage. Microbe Damage - Has an unbroken surface and “acid mantle” (skin secretions are acidic and thus inhibit microbes, such as bacteria). Phagocytes (Langerhans Cells) ingest foreign substances and pathogens, preventing them from penetrating deeper body tissues. Ultraviolet (UV) radiation (Damaging effects of sunlight or tanning beds) - Melanin produced by melanocytes offers protection from UV damage. Thermal (heat or cold) damage - Contains heat/cold/pain receptors. Desiccation (drying out) - Contains a water-resistant glycolipid and keratin. Aids in body heat loss or heat retention (controlled by the nervous system) - Heat loss: By activating sweat glands and by allowing blood to flush into skin capillary beds so that heat can radiate from the skin surface. Heat Retention: By not allowing blood to flush into skin capillary beds. Aids in excretion of urea and uric acid - Contained in perspiration produced by sweat glands Synthesizes vitamin D - Modified cholesterol molecules in skin converted to vitamin D in the presence of sunlight | The integumentary system is responsible for keeping the boundaries of the body and maintaining homeostasis. The organs and tissues included in this system include the: Skin Hair Fingernails Glands The Skin The skin has multiple layers that protect the body, regulate body temperature, and excrete excess waste. The skin is the largest organ in the body. Layers of the skin Epidermis
Stratum Corneum
Stratum Lucidum
Stratum Granulosum
Stratum Spinosum
Stratum Basale
Dermis
Hypodermis
|
The Skeletal System
Short Bones: Roughly cube-shaped bones with the same length, width, and thickness dimensions. Long Bones: Long dense bones that have a shaft and two ends. Joints: Where two bones are connected. Important Bones Skull (Scientific name: Cranium) Collarbone (Sciencetific Name: Clavical) Breastbone (Scientific Name: Sternum) Upper Leg Bone (Scientific Name: Femur) Lower Leg Bone (Scientific Name: Tibia) Upper Arm Bone (Scientific Name: Humerus) Lower Arm Bone (Scientific Name: Radius) Backbone (Scientific Name: Vertebrae) Osteoclast: A type of cell that breaks down bone to release calcium into the blood. Osteoblast: A type of cell that builds new bone. Osteocyte: A type of bone cell that connects to other osteocytes to form bone tissue.Con | Skeleton Anatomy The skeleton is split into two sections, the axial skeleton and the appendicular skeleton. Axial Skeleton
Spinal Column The spinal column or vertebral column consists of 33 vertebrae (Some vertebrae fuse as you age). There is also an intervertebral disk between most vertebrae. The first vertebrae is called the atlas and the second is called the axis. The first 7 are called the cervical vertebrae. The next 12 are called the thoracic vertebrae. The next five are the lumbar vertebrae. The last 10 are called the sacral and coccyx vertebrae (These vertebrae fused to create 1 sacrum and 1 coccyx). As you go down the spinal column, the vertebrae get thicker. Thus in size and thickness: Cervical < Thoracic < Lumbar < Sacral Rib Cage Consists of 24 ribs, each making 12 pairs. Classification of ribs
Appendicular Skeleton
Shoulder Girdle Comprised four bones, two clavicle bones, and 2 scapulas. Arm Comprised of three bones, the upper bone (humerus), the radius, and the ulna. At the bottom of the arms are the hand bones. They are comprised of
Pelvic Girdle This is the structure in which the leg bones are attached. It is comprised of 2 ox coxae (hip bones). Each hip bone is divided into three sections, the ilium, the ischium, and the symphysis (pubic bone). Leg Bone These bones are attached to the pelvic sockets (acetabulums). Each leg is comprised of one femur, one fibula (shin bone), and one tibia. There is also an in-between bone called the patella (knee cap). At the bottom of the legs are the feet’ bones They are comprised of
Skeleton Physiology The skeletal system is tasked with keeping the body structured and intact. They give the body the structure for it to move. The skeleton protects the important organs of the body from damage and trauma. Spinal Column
Rib Cage
Long Bone Anatomy The two ends located on a long bone are called epiphyses. The top epiphysis is called the proximal epiphysis and the other one is called the distal epiphysis. The shaft of the long bone is called the diaphysis. This part of the bone gives support and leverage for movement. The outer shell of the long bone is made of cortical bone (compact bone). Beneath that layer is the cancellous bone. Short Bone Anatomy Short bones are also made up of cortical and cancellous tissue. Long Bone Physiology Long bones support the body’s load during daily activities. They are essential for the mobility of the skeleton. Short Bone Physiology Support areas with a lot of physical activity or areas that need extra protection. Osteoclasts, Osteoblasts, and Osteocytes Osteoclasts
Osteoblasts
Osteocytes
|
The Endocrine System
Endocrine System: The organ system that produces, releases, and reabsorbs hormones. Hormones: A regulatory substance that controls the actions of target cells. Cascades: When hormones trigger the release or power the effect of other hormones. Gland: Any structure that makes and secretes hormones. Target Cell: Any cell that a specific hormone is intended for. Types of Hormones and Their Functions Adrenaline: Increase systolic blood pressure, glycogenolysis, lipolysis, increase cardiac output, influence goosebumps, etc. Melatonin: Managing sleep–wake cycle Dopamine: Regulation of cellular cAMP levels, prolactin antagonist (gives feelings of pleasure and satisfaction) | Hormones control almost all cells in the body. They regulate:
The endocrine system is similar in ways to the nervous system. Both systems monitor and respond to changes within the body, just in different ways. Organs of the Endocrine System The endocrine system is made up of glands that produce and release the hormones. The main gland is the pituitary gland. There are also a few organs associated with this system. Pituitary Gland This gland produces a plethora of hormones that trigger other glands like the thyroid, parathyroid, adrenal, and pineal glands to release their hormones. Hormones Hormones can only trigger reactions in specific cells. These are called the target cells. Chemically, most hormones are made up of amino acids. Others are made from lipids. This structure determines if the hormone is water soluble or lipid soluble. Water-soluble hormones cannot get across the cell membrane by themselves. Target cells for these hormones have receptors on the surface of the membrane that bind to their specific hormone. Lipophilic hormones can “glide” across the cell membrane, so their receptors are located inside the target cell. The hormone alters the physiology of the target cell once it has activated it. Some of the cell functions are either increased or decreased. |
Subject: Health
Vitamins
Vitamin: Organic compounds that are required for growth and nutrition and are required in small quantities. Fat Soluble Vitamins: Vitamins that dissolve in fats and oils and are stored in the body’s fatty tissue and liver. (Fat-soluble vitamins are hydrophobic and lipophilic) Water Soluble Vitamins: Vitamins that are dissolved in water and carried to the body tissue (They are not stored in the body). Vitamin A (Retinol, Beta-Carotene): A fat soluble vitamin that is related to retinol. Vitamin D (Calciferol): A fat-soluble vitamin that is synthesized by the skin. Vitamin E (Phylloquinone, Menaquinone): A group of eight related compounds in molecule structure that include four tocopherols and four tocotrienols. | Fat Soluble Vitamins: Vitamin A
Vitamin D
Vitamin E
Vitamin K
Water-Soluble Vitamins Vitamin B1
Vitamin B2
Vitamin B3 (Niacin)
Vitamin B5 (Pantothenic Acid)
Vitamin B6 (Pyridoxine)
Vitamin B7 (Biotin)
Vitamin B9 (Folate/Folic Acid)
Vitamin B12 (Cobalamin)
Vitamin C (Ascorbic Acid)
|
Chemistry
Subject: Nomenclature
Naming Ionic Compounds
Polyatomic Ion: An ionic compound with more than one atom. Binary Molecular Compound: A compound composed of two elements Binary Acid: Typically two elements Oxyacid: Acid with an oxyanion | Identifying Compounds Ionic compounds are usually a combination of a metal with a nonmetal. Molecular compounds are usually made of nonmetals. Naming Ionic Compounds Nonmetals tend to gain electrons to get more stable while metals tend to lose electrons. The Ionic compound must be in a form that results in neutrality. Steps:
Example: NaCl = 0 Na = Cation Cl = Anion NaCl = Sodium Chloride (Group 1 and 2 metals can take on multiple changes (this makes naming more difficult)) For these types of compounds, we put a Roman numeral in parentheses after the metal. We place the numeral as its oxidation state. There are four metals that we do not give a Roman numeral: Ag (Silver), Al (Aluminum), Cd (Cadmium), and Zn (Zinc). There are also common names for the different oxidation states of the Roman numeral metals. Example: Copper (I) = Cuprous Copper (II) = Cupric |
Subject: Nuclear Chemistry
Nuclear Chemistry: The study of changes to the nucleus of atoms. Isotopes: Atoms of the same element that have different numbers of neutrons. Transmutation: The change of one isotope or atom to another. Radioactivity: The process of radioactive decay. Half-Life: The time it takes for one-half of a radioactive sample to decay. Ionizing Radiation: The release of energy that allows stability. (It is ionizing because it has the power to knock electrons out or add electrons to other atoms.) Alpha Decay: A type of decay that releases an alpha particle Beta Decay: A type of decay that only releases an electron. Gamma Decay: A type of decay that only releases electromagnetic energy. Excited State: The energy level of an electron that is higher and less stable. Ground State: The lowest most stable energy level of an electron. Spontaneous Fission: Radioactivity when an atom’s nucleus breaks in two. | Reactions happen due to the interaction of electrons. However, protons and neutrons can sometimes interact, releasing energy. Any change to the number of protons will change an atom into a different one. When the nucleus of an atom isn’t stable, protons and neutrons are released to reach stability. Fun Fact: Carbon 14 is a radioactive isotope that is constantly renewed by cosmic rays in Earth’s atmosphere. Radioactive decay occurs when a nucleus has more energy than a more stable version. When decay occurs, ionizing radiation is released as the energy byproduct. Types of Decay
The alpha particle is basically the same as a helium nucleus. a Particle = Helium-4 Nucleus = 24He The alpha particle is two protons and two neutrons. Example: 23892U to 24He + 23490Th
B Particle = Electrons = 0-1e Beta Decay has higher energy than alpha decay. Example: 23490Th to 0-1e + 23491Xe
Y = High-Frequency Electromagnetic Energy = 00Y Gamma decay usually happens when electrons transition from an excited state to a ground state. Gamma rays are the strongest of radiation. Example: 6028Ni* to 00Y+ 6028Ni |
Subject: Types of Chemical Reactions
Combustion Reaction
Combustion Reaction: A reaction in which a reactant reacts with water and produces a high amount of energy as a result. Synthesis Reaction: A reaction in which two or more reactants combine to form one compound. Decomposition Reaction: A reaction in which a complex compound is broken into the components that created said compound. | Combustion Reaction Combustion reactions usually involve burning and they release a lot of heat energy. These types of reactions are good at making work and heat. They are commonly used for energy in cars, planes, and factories. Example: Octane (C8H8) + Oxygen (O2) = Carbon Dioxide (CO2) + Water (H2O). (Since the reaction is so hot, both the products are released as gases). When carbon dioxide and water are products of a reaction, you can tell it is a combustion reaction. Synthesis Reaction Synthesis reactions are combination reactions. They end in one larger compound or thing being produced. Example: Mg + O2 = MgO2 Decomposition Reaction The type of reaction that breaks apart complex objects into less complex components. This is the reverse of a synthesis reaction. Adding heat commonly breaks apart compounds. Single replacement reaction A + BC = AC + B • Just as the name says, a single molecule from the same equation replaces another. For the most part, metals tend to replace metals and nonmetals tend to replace nonmetals. All "Single Replacement" reactions are "Redox Reactions". Double Replacement Reaction AB + CD = AD + BC similar to the Single replacement reaction; however, this one is double, and completely switches elements' places up. Both of the reactants must be aqueous When a double replacement happens between two aqueous solutions and the products all remain aqueous then no reaction takes place. A "Double Replacement" reaction is NEVER a "Redox reaction" Double Replacement reaction types: - If a double replacement of two aqueous products happens and you find a solid in the product, then it's called a "Precipitation Reaction". - If a double replacement happens between a strong acid and a strong base, it will produce water and salt and will be called "Acid-Base Neutralisation Reaction". - If a double replacement of two aqueous products happens and you find a gas in the product, then it's called a "Gas Evolution Reaction". Redox Reactions Also called "oxidation-reduction reaction", is any chemical reaction in which the oxidation number of atoms changes. -Methane(CH4) is also called: Natural gas. -Almost all ionic compounds are considered salts. |
Subject: Chemical Formulas and Chemical Compounds
Chemical Names and Formulas
Subject: Arrangements of Electrons in Atoms
Electromagnetic Radiation: A form of energy that exhibits wavelike behavior as it travels through space. Electromagnetic Spectrum: All the forms of electromagnetic radiation. Wavelength: The distance between corresponding points on adjacent waves (a distance unit). Frequency: The number of waves that pass a given point in a specific time (usually one second) (It is expressed in waves per second, one wave/sec is called a hertz (Hz)). Photoelectric Effect: The emission of electrons from a metal when light shines on the metal. Quantum: The minimum quantity of energy that can be lost or gained by an atom. | Wave Description of Light Visible light is a form of electromagnetic radiation. Other kinds include x-rays, ultraviolet, and infrared light. All forms of electromagnetic radiation move at a constant speed of 3.00 x 108 m/s through a vacuum and slightly slower through matter. (3.00 × 10^8 is also approximately the speed of light through air). In electromagnetic radiation, the mathematical relationship between frequency and wavelength is written as follows: c = ʎV In this equation, c is the speed of light (in m/s), ʎ is the wavelength of the electromagnetic wave (in m), and V is the frequency of the electromagnetic wave (in s-1). Because c is the same for all electromagnetic radiation, the product ʎV is a constant. New Atomic Model |
Physics
Subject: Particle Physics
The Standard Model
Fermion: Particles that make up the physical matter of the universe and are located to the left of the standard model. Bosons: Particles that mediate how matter particles behave (force carriers or exchange particles) Spin: A force of angular momentum in an angular motion (intrinsic angular momentum) Conservation Laws of Particle Physics: The ways particles must interact with each other. Pauli Exclusion Principle: Fermions can’t share the same quantum state. Boson Statistics: The term given to describe the ability of bosons to take up the same quantum states. Composite Bosons: Things that behave like bosons due to spin conservation. Quarks: Particles that don’t exist on their own and carry an electric charge. Baryon Number: The number of baryons in a particle. Baryon: Any particle made of an odd number of three or more quarks (Example: Neutrons and Protons). Anti-Particle: A particle that has the opposite values of a fundamental particle except for its mass. Color Charge: A charge similar to the electric charge, but with three charge states. Lepton Flavors: The different quantum numbers of each neutrino
Neutrino Chirality: The direction of their chirality which is left. | The Standard Model shows all the discovered fundamental particles. The main difference between bosons and fermions is their spin. Fermions have a spin of ½ while bosons have a spin of 1 (0 in the case of the Higgs boson) Conservation Laws The spin must be conserved. In particle interactions, spin will always remain constant. Energy and linear motion are the most fundamental conservation laws. Fermions in a group obey the Pauli Exclusion Principle Bosons are opposite as they can take on the same quantum state. Bosons are anything with a spin of a whole integer. Fermions are anything with a spin of a decimal integer Fermions Fermions are split into two categories, quarks and leptons. Quarks; Up and down Quarks make up neutrons and protons. The resulting charges of the particles are the sum of the fundamental quark charges. Up = 2/3 Charm = 2/3 Top = 2/3 Down = -1/3 Strange = -1/3 Bottom = -1/3 Quarks interact with all of the forces. They are also the only particles that feel the strong nuclear force. All quarks and gluons have a color charge. Quarks are restricted from coming together unless they have color charges that result in a color-neutral particle. Leptons; Electrons are the best leptons as they are responsible for chemical reactions and electricity. Leptons are made up of two groups, the neutrinos and the charged leptons. Neutrino Properties Neutrinos only interact with the weak force and barely interact with matter. Neutrinos have a very small mass greater than zero and all their masses combine to 0.3 cV. Neutrinos can change between lepton flavors. Neutrinos have only been observed to only be left-handed. Anti-Neutrinos are only right-handed. |
Dark Matter
Dark Matter: A hypothetical substance that makes up most of the matter in galaxies. Axion: Subatomic particles that have not been discovered but have properties of dark matter. Dark Energy: A theoretical force that repulses all matter in the universe and counteracts gravity. | The universe as we know it is only made up of 5% atoms and molecules. Another 25% of the universe is made up of dark matter. The rest 70% is made up of dark energy. Dark matter affects the gravitational pull of galaxies, as we theorized. Scientists observed a constant orbit speed throughout multiple galaxies, which contradicts the theories of gravity. Therefore, a theoretical substance that could not be observed, dark matter, was hypothesized. |
Light
Huygens Principle: A principle that states that you can predict the position of a wave in the future by looking at its current position. Diffraction: The process in which waves are re-shaped by obstacles. Double Slit Experiment: An experiment to see if light was a particle or a wave. Intensity: The energy transported by the light over time or its brightness. 1 = (energy/time)/area = power/area | Is light a wave or a particle? In early physics, light was believed to be a particle. Now, we know that light is both a wave and a particle. r=vt Waves behave in a set of ways that allow us to observe them. All waves follow the Huygens Principle, but when an object interferes, the wavelets are changed. Particles move straight meaning that they don’t end up behind the object. Double Slit Experiment A window was opened with a double-sided slit behind it. Behind that slit was two more slits. If light were a particle, there would be two bright spots. Instead, there were multiple lines on the screen. This meant that light was a wave. The diffraction pattern was created by the light interference. In slit experiments where the slits were an exact wavelength apart and have constructive interference. If they were not, they would interfere destructively. Path Difference Equation d x sinO = p P equals the distance of each slit multiplied by the angle’s sine between the screens point and the straight line between the slits and the screen. Light with more energy has a shorter wavelength (more on the blue side), while light with less energy has a longer wavelength (on the redder side). Depending on the angle, different rays travel different distances. Depending on how they line up, the rays interact constructively or destructively. With a total path difference of a full wavelength, for each light ray, there’s a corresponding light ray that’s shifted by half a wavelength. This causes destructive interference of the two rays. |
Subject: Mechanics
Kinematics
Kinematics: The study of motion without considering the forces causing it. Average speed considers total distance over time, while average velocity focuses on displacement over time. Acceleration measures the rate of change in velocity. Constant velocity implies zero acceleration. Projectile motion exhibits a parabolic trajectory due to the independent horizontal and vertical components of motion. Horizontal motion: The motion of the object in the x direction is constant and is not affected by gravity. Vertical motion: The motion of the object in the y direction is affected by gravity and follows a parabolic path. Free fall signifies motion under the sole influence of gravity, with all objects experiencing the same acceleration regardless of mass. Terminal velocity marks the maximum speed an object attains during free fall due to air resistance. | Kinematics involves analyzing distance, displacement, speed, velocity, and acceleration. Importantly, while distance is the total path length, displacement focuses solely on the change in position. Similarly, speed is scalar, representing the rate of distance covered, while velocity is a vector encompassing both the rate and direction of displacement. Important Equations of Kinematics
These are equations that describe movement in one and two dimensions where acceleration is constant. Two Dimensional Motion In 2-D, project motion is introduced. Gravity |
Work, Energy, and Power
System: A section of the universe that you happen to be talking about at a certain time. Work: The force applied to a system for a certain distance. Power: Energy: The ability to do work. Kinetic Energy: The energy of motion Potential Energy: Energy that could be used to do work. | Work If you wanted to pull a box, your pulling would be the force and the box would be the system. The amount of work you did to pull the box would be the force times the distance the box moved. If the force put in is 50N And the distance traveled was 5m Then the work would be 250J If the example included a force that was not in the same direction as the movement of the box, we would calculate a little differently. In an instance where the rope pulling the box was higher than the box, the force would be an angle to the box. To calculate the work, we would multiply the distance by the cos Thus W = F(d)(cos To find the work done by varying distances in a case where the force is not constant, you must integrate the force relative to the distance the object moved. Thus in this case W = ∫ F(x)dx (assuming force is parallel to distance moved) Work can be thought of as the change of energy. Kinetic and Potential Energy Kinetic energy is activated when work is applied KE = 1/2m v² Potential energy is the possible work that can be applied. The most common example of potential energy is gravitational potential energy. When an object falls, gravity does work on the object and pulls it down. Once it reaches the ground, however, its gravitational potential energy is zero. PEgravity |