Summary of other drugs
Summary ✨ other drugs ✨
Antifungal drugs
- Fungi can have both sexual and asexual reproduction in the same organism; budding, spore formation happens in the same fungi!
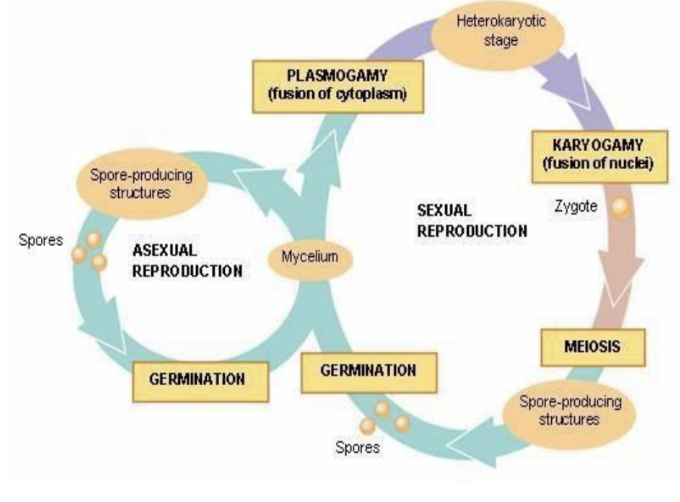
- This is one of the reasons as to why they’re so successful!
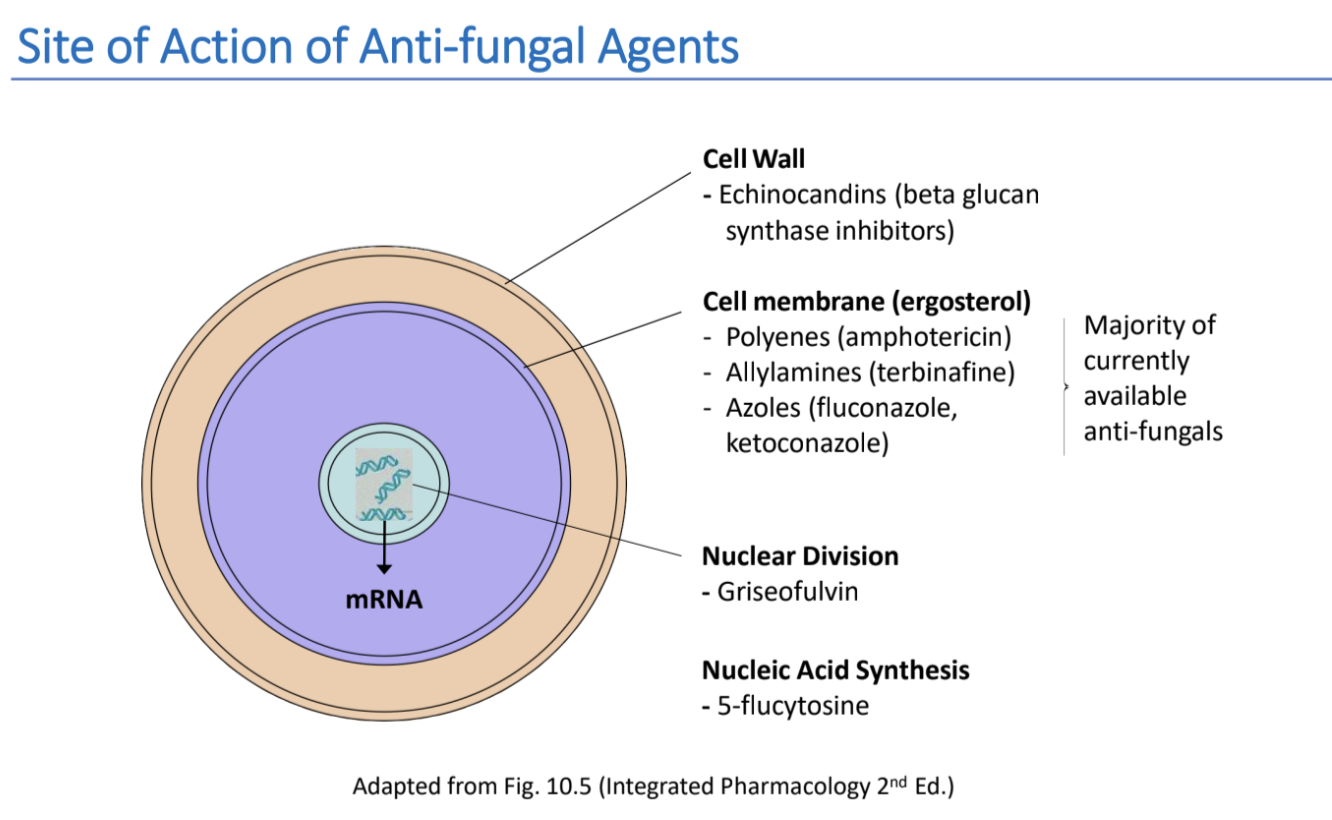
Targets for fungi
There are three sites of targets for anti-fungal drugs:
- Cell wall - where the drug inhibit cell wall synthesis by inhibiting the enzyme beta-(1,3)-d-glucan synthase. It is responsible for catalysing beta-(1,3)-d-glucan, which is an essential part of the cell wall.
- Cell membrane - where the drug targets ergosterol. It has functions which are very similar to cholesterol in humans, and fungi cannot survive without it. It is found in the cell membrane. The steps of synthesising is squalene -> lanosterol -> ergosterol.
- Nucleus - where drugs either target nuclear division, by disrupting the mitotic spindle, or inhibiting DNA replication by inhibiting thymidylate synthase.
Cell wall: Echinocandins
Echinocandins are drugs which inhibit cell wall synthesis. They are irreversible inhibitors of beta-(1,3)-d-glucan synthase, which is the enzyme that is needed to synthesize beta(1,3)-d-glucan: an essential part of the cell wall.
Caspofungin
- Clinical use: invasive candidiasis, suspected fungal infections in febrile neutropenic patients, aspergillus infections in patients who are nonresponsive to amphotericin.
- Pharmacokinetics: Slow IV infusion, widely distributed, highly protein bound, hepatic clerance
- Side effects: well tolerated, can cause headaches, fever, raised liver function tests
Micafungin
- Clinical use: invasive candidiasis, prophylaxis against Candida & Apergillus species in patients getting haemopoietic stem cell transplantation.
- Pharmacokinetics: Highly protein bound
- Side effects: Shouldn’t be given to people with liver damage
Cell membrane
There are three types of groups which target the cell membrane, however they all have in common that they target the sterol ergosterol. This is found in the cell membrane of fungi and the fungi cannot survive without ergosterol.
- Ergosterol polyenes are a group which binds ergosterol and therefore forms pores in the membrane. An example is Amphotericin B.
- Ergosterol azoles inhibit ergosterol synthesis. Examples are Fluconazole, Ketoconazole.
- Ergosterol allylazmines inhibit ergosterol synthesis and build-up of fungicidal intermediary (squalene). An example is Terbinafine.
Amphotericin B
- Type of ergosterol polyene (A)
- MoA: Binds to ergosterole in cell membranes, which forms a pore in the membrane. This will cause loss of intracellular K+ ions and macromolecules, and lead to cell death.
- Clinical uses: wide spectrum against systemic infections
- Pharmacokinetics: IV or topical
- Side effects: Can cause renal toxicity in 80%!!! or hypokalemia in 25%... Therefore Liposomal Amphotericin B is used instead, which has less SE.
Fluconazole
- Is an ergosterol azoles (B)
- MoA: Inhibits fungal oxidative enzymes, which will cause a lethal accumulation of hydrogen peroxide
- It also inhibits cytochrome P450 3a enzymes, which will prevent the conversion of Lanosterol into Ergosterol
- Pharmacokinetics: Oral is really good, can be IV for system infections
- Clinical: cryptococcal meningitis, prophylaxis for a variety of conditions
- Side effects: well tolerated, GIT discomfort, headaches, allergic rashes
Ketoconazole
- Is an ergosterol azoles, same MoA as Fluconazole (B)
- Pharmacokinetics: good orally, no CNS
- SE: Hepatotoxic, GIT disturbances, endocrine effects, drug interactions (use fluconazole instead)
Terbinafine
- Is an ergosterol allylamine (C)
- MoA: Interferes with ergosterol biosynthesis and therefore the formation of the fungal cell membrane. It inhibits squalene epoxidase, which turns squaline into lanosterol. This will decrease ergosterol synthesis, but increase squalene which is fungicidal!!
- Pharmacokinetics: Oral; Well absorbed in GIT, metabolized in the liver
- Clinical uses: Topically for dermatophyte infections of the skin, orally for infections of hair and nails
- SE: very mild, nasuea, abdominal pains, GIT disturbances, headaches
Nucleus
There are two types of drugs which target the nucleus: Nuclear division (griseofulvin) and DNA replication (Flucytosine).
Griseofulvin
- MoA: Binds to polymerised microtubulues, therefore disrupting the mitotic spindle and blocking replication in mitosis.
- Pharmacokinetics: Oral administration, taken up by keratin in skin and nails, induces cyt p450 activity.
- Clinical uses: Narrow spectrum, fungistatic. Prolonged treatment for skin and nail infections.
- Side effects: Mild, GIT disturbances, headaches, photosensitivity, allergi.
Flucytosine
- MoA: It requires cytosine permease to enter the cell, which is only expressed on fungal cells. It is then converted to 5-fluoruracil by cytosine deaminase. It then inhibits thymidylate synthase which leads to inhibition of DNA synthesis.
- Clinical uses: Is mostly used for yeast and cryptococcal meningitis. Narrow spectrum.
- Pharmacokinetics: IV, oral, widely distributed (including CSF).
- SE: Mild: GIT disturbances, anaemia, decreased neutrophils in the blood
- Resistance: will occur due to decreased levels of converting enzymes in fungus, loss of permease for cytosine transport.
Anti-protozoal drugs
Protozoa
Protozoa are eukaryotic, unicellular organisms with motile, diverse life cycles. There are more than 50,000 species! They can cause infections that are all from asymptomatic to life threatening. Protozoa infect host tissues/organs either as an intracellular or extracellular parasite. They are divided into classes based on their locomotion:
- Amoebae (e.g. Entamoeba)
- Flagellates (e.g. leishmania)
- Ciliates (e.g. balantidium)
- Sporozoa (e.g. plasmodium)
They are commonly transmitted through one of the two methods:
- Vector-borne: Injection via a bite of blood-sucking insects
- Fecal-oral: Accidental ingestion of infective stages
Malaria
Is a disease which is caused by protozoa, usually vector-borne transmission by mosquitoes. It is most commonly caused by plasmodium falciparum, P.vivax, P.malariae and P.ovale.
Symptoms of malaria appears a week after infection, and vary from mild like fever, headache, vomiting to severe, such as anaemmia, kidney failure, respiratory distress, coma and death (Y). The symptoms are often cyclical, meaning that you’ll have fever, then chills, then fever again and circle around.
Life cycle of the plasmodium (malaria parasite)
- Sporozoite infect human through mosquito bite
- Sporozoite travels through blood stream and infects hepatocytes in the liver
- Turns into schizoid where there are division and maturation
- Hepatocyte ruptures and releases merozoites
- Merozoites moves into the bloodstream and starts infiltrating red blood cells,
- Trophozoites are formed in the red blood cells, which matures and starts infiltrating the red blood cells by inserting parasite protein and phospholipids into the cell membrane, and digesting the hemoglobin.
- The red blood cells turn into schizoid;
- The red blood cells ruptures and releases merozoites which will infiltrate more red blood cells
- Some of the parasites differentiate into gametocytes, which are either female or male.
- The gametocytes are taken up by mosquitos when they feed
- The gametocytes turn into zygotes in the mosquitos stomach
- The zygote turns into an oocyte sporocyst
- The oocyte sporocyst ruptures; releasing sporozoites
- The mosquitoes infect humans through mosquito bites.
→ Obs! P. Vivax have resting forms called Hypnozoites, which are in the liver and can last for months/years and may continue to relapse post-therapy…
→ Obs! Plasmodium Falciparum is the most severe one and that one skips the liver phase, and the infected RBCs can stick to the vascular endothelial cells.
Drugs which tackle malaria
There are four classes of drugs which tackles malaria:
- Drugs used to treat the acute attack (sporozoites in blood) (Quinine)
- Drugs which target the parasites in the liver (attack schizonts in liver) (primaquine)
- Drugs which target the step linking tissue and blood
- Drugs which prevent transmission (gametocytes)
A. Drugs used to treat the acute attack
These drugs are blood schizonticidal agents. The affect the symptoms, but not the cause. There are several ones used, often in combination:
- 4-aminoquinolines, e.g. chloroquinine
- Quinoline-methanol: Quinine
- Phenanthrenene-methanols, e.g. halofantrine
- Folate anti-metabolites: e.g. sulphones
- Some antibiotics, e.g. tetracycline
Quinine
- Is a type of quinoline-methanol
- It’s an alkaloid derived from cinchona bark
- MoA: Is a bit unclear, but it might utilise parasite-specific drug concentrating mechanisms. It could also be so that it inhibits haem polymerase: which will make sure that haem is not formed into haemozoin. Free haem is very toxic to the parasites, which is why they turn it into haemozoin. Therefore would inhibition of haem polymerase be toxic.
- Pharmacokinetics: Orally, high tissue distribution especially in liver, spleen, lung and kidney. Metabolised in liver.
- Clinical use: Used for multidrug resistant plasmodia species
- SE: Nausea, vomiting, dizziness, tinnitus, fever, hypoglycemia
B. Drugs which target parasites in the liver
Drugs in this class act on the schizonts in the liver, but also destroyed gametocytes and therefore reduce the spread of infection.
Primaquine
- Is a type of 4-aminoquinoline, so it is also used to treat the acute attack.
- Therapeutic uses: most active against P. Falciparum, effective against hypnozoites and destroys sexual forms of all plasmodium species!
- MoA: Unclear but through to be in a two step way:
- Primaquine is turned into an hydroxylated metabolite (OH-PQm)
- Metabolite undergoes spontaneous oxidation into quinoeimines which generates H2O2 → which will kill the plasmodium parasites
- Pharmacokinetics: oral
- Side effects: GIT disturbances, can cause dangerous side effects in people who are deficient in glucose-6-phosphate dehydrogenase enzyme: which normally helps to defend red blood cells against stress. This is because primaquine makes the RBC to bursts and in people with g-6-p deficiency, this will lead to anaemia.
C. Drugs which block the link between exo-erythrocytic and erythrocytic stages
These are drug which makes sure that the …
They are administrated 1 week prior to travel, as to stop the movement of the protezoa.
Examples are:
- chloroquine, which is a type of 4-aminoquinoline with the same MoA as quinine.
- Doxycycline
- Folate anti-metabolites, e.g. pyrimethamine
- Mefloquine
- Proguanil
Chloroquine
- Is a type of quinoline analouge
- Type of 4-aminoquinoline
- MoA: Is a bit unclear, but it might utilise parasite-specific drug concentrating mechanisms. It could also be so that it inhibits haem polymerase: which will make sure that haem is not formed into haemozoin. Free haem is very toxic to the parasites, which is why they turn it into haemozoin. Therefore would inhibition of haem polymerase be toxic.
- Pharmacokinetics: Orally, high tissue distribution especially in liver, spleen, lung and kidney. Metabolised in liver.
- Clinical use: effective against eryhtocytic forms of all four plasmodium species, unless they are resistant. Used for blood schizonticides (class A&C?) and gametocidal (class C).
- SE: Nausea, vomiting, dizziness, headache, hives, photosensitivity. Might cause organ system effects, such as depress myocardium, cause insomnia, and retinopathy.
- Resistance: is caused due to increased efflux or decreased uptake.
D. Blockage of transmission (targeting gametocytes)
These drugs are important to prevent the transmission of the parasite, however they are very seldom used for this action alone.
Proguanil, pyrimethamine and primaquine are used for this.