Moles notes
Key vocab:
Species: A molecule, atom, ion or compound in an equation
Mole: The amount of substance. The unit is moles or mol
Relative atomic mass: The mass found on the periodic table for a element (The mass of an atom which is the weighted average mass of all isotopes of the element, relative to 1/12 the ass of an atom carbon of 12)
Relative formula mass, Mr: he sum of all the atomic masses of atoms represented in a chemical formula
Relative molecular mass Mr: A term for formula mass that can be used with molecules
Empirical formula: Shows the simplest whole number ratio of the atoms of different elements present in a compound
Relative Atomic Mass (Ar) and Relative Formula Mass (Mr)
Relative Atomic Mass (Ar):
The relative atomic mass of an element (Ar) is the weighted average mass of its atoms compared to 1/12th of the mass of a carbon-12 atom (which is defined as exactly 12 units). It considers the natural abundance of the element’s isotopes.
Example: The Ar of oxygen is approximately 16, while the Ar of hydrogen is about 1.
The periodic table provides the Ar values for each element, which can then be used to calculate the masses of compounds.
Relative Formula Mass (Mr):
The relative formula mass (Mr) is the sum of the relative atomic masses of all atoms in a chemical formula.
If dealing with a molecule, Mr is often called the relative molecular mass.
Example:
For water (H₂O): Mr = (2 × Ar of H) + (1 × Ar of O) = (2 × 1) + (16) = 18.
For sodium chloride (NaCl): Mr = (1 × Ar of Na) + (1 × Ar of Cl) = 23 + 35.5 = 58.5.
Importance: Mr is used in chemical calculations to convert between mass and moles, and for determining molar concentrations in solutions.
The Mole and Amount of Substance
Mole (mol):
The mole is the SI unit for the amount of substance. 1 mole of any substance contains 6.02 × 10²³ particles (atoms, molecules, ions, etc.), known as Avogadro's number.
The mass of 1 mole of a substance in grams is equal to its relative formula mass (Mr). For instance, 1 mole of water (H₂O) weighs 18 g because the Mr of water is 18.
Amount of Substance Calculation:
To calculate the amount of substance, use the formula:
Amount of substance (mol) = mass (g) ÷ Mr.Example:
If you have 36 g of water (H₂O), and the Mr of H₂O is 18:
Moles of water = mass ÷ Mr = 36 g ÷ 18 = 2 mol.
This formula is fundamental in stoichiometry for working out the quantities of reactants and products in chemical reactions.
Formulae of Simple Compounds
Obtaining formulae experimentally:
The formula of a compound tells you the simplest ratio of the elements involved. For instance, in magnesium oxide (MgO), the formula indicates a 1:1 ratio of magnesium atoms to oxygen atoms.
Experimental data can help deduce this ratio for compounds like:
Metal Oxides: Metals react with oxygen to form oxides. By measuring the mass of the metal and the mass of the oxygen it reacts with, the formula can be deduced.
Water of Crystallisation in Salts: Some salts contain water molecules within their crystal structure, known as water of crystallisation. The formula of a hydrated salt (e.g., CuSO₄∙5H₂O) can be determined by heating the salt and measuring the loss in mass as the water evaporates. The ratio of water to salt helps determine the hydrated formula.
Empirical and Molecular Formula
Empirical Formula:
The empirical formula represents the simplest whole-number ratio of atoms of each element in a compound. It doesn't necessarily show the exact number of atoms in a molecule, just the ratio.
For example, the empirical formula of glucose (C₆H₁₂O₆) is CH₂O, showing a 1:2:1 ratio of carbon, hydrogen, and oxygen.
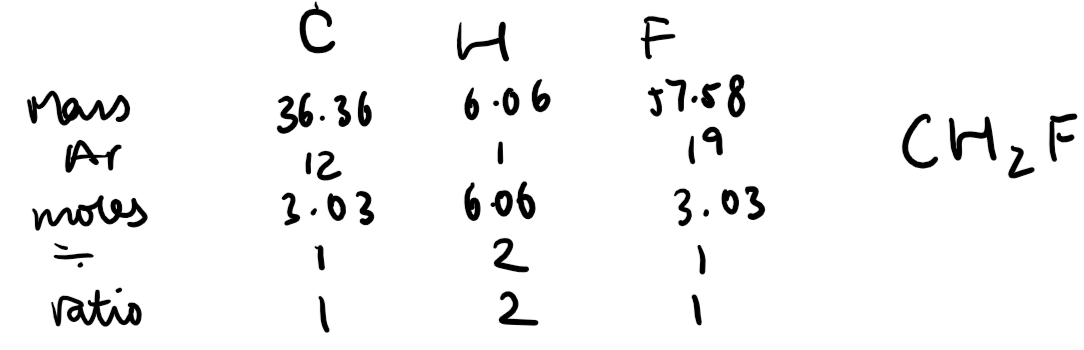
Molecular Formula:
The molecular formula shows the actual number of atoms of each element in a molecule.
For example, the molecular formula of glucose is C₆H₁₂O₆, meaning each molecule contains 6 carbon, 12 hydrogen, and 6 oxygen atoms.
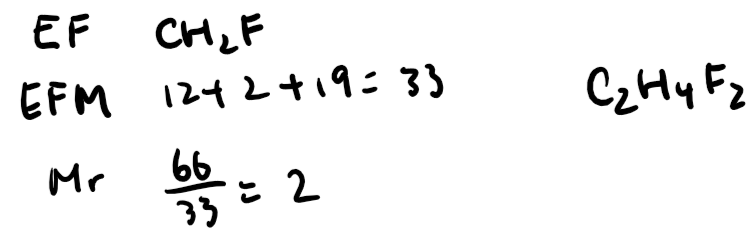
Calculation:
Empirical Formula:
From experimental data, the mass or percentage composition of each element in a compound can be converted into moles (mass ÷ Ar).
The simplest whole-number ratio of the moles gives the empirical formula.
Example: A compound contains 40% sulfur and 60% oxygen. The Ar of sulfur is 32, and the Ar of oxygen is 16.
Moles of sulfur = 40 ÷ 32 = 1.25 mol.
Moles of oxygen = 60 ÷ 16 = 3.75 mol.
Simplifying the ratio (1.25:3.75) gives 1:3, so the empirical formula is SO₃.
Molecular Formula:
The molecular formula is a multiple of the empirical formula. To find it:
Calculate the Mr of the empirical formula.
Divide the molecular Mr (given) by the Mr of the empirical formula to find a whole number.
Multiply the empirical formula by this number to get the molecular formula.
Example: If the empirical formula is CH₂, and the molecular Mr is 28, the molecular formula is C₂H₄.
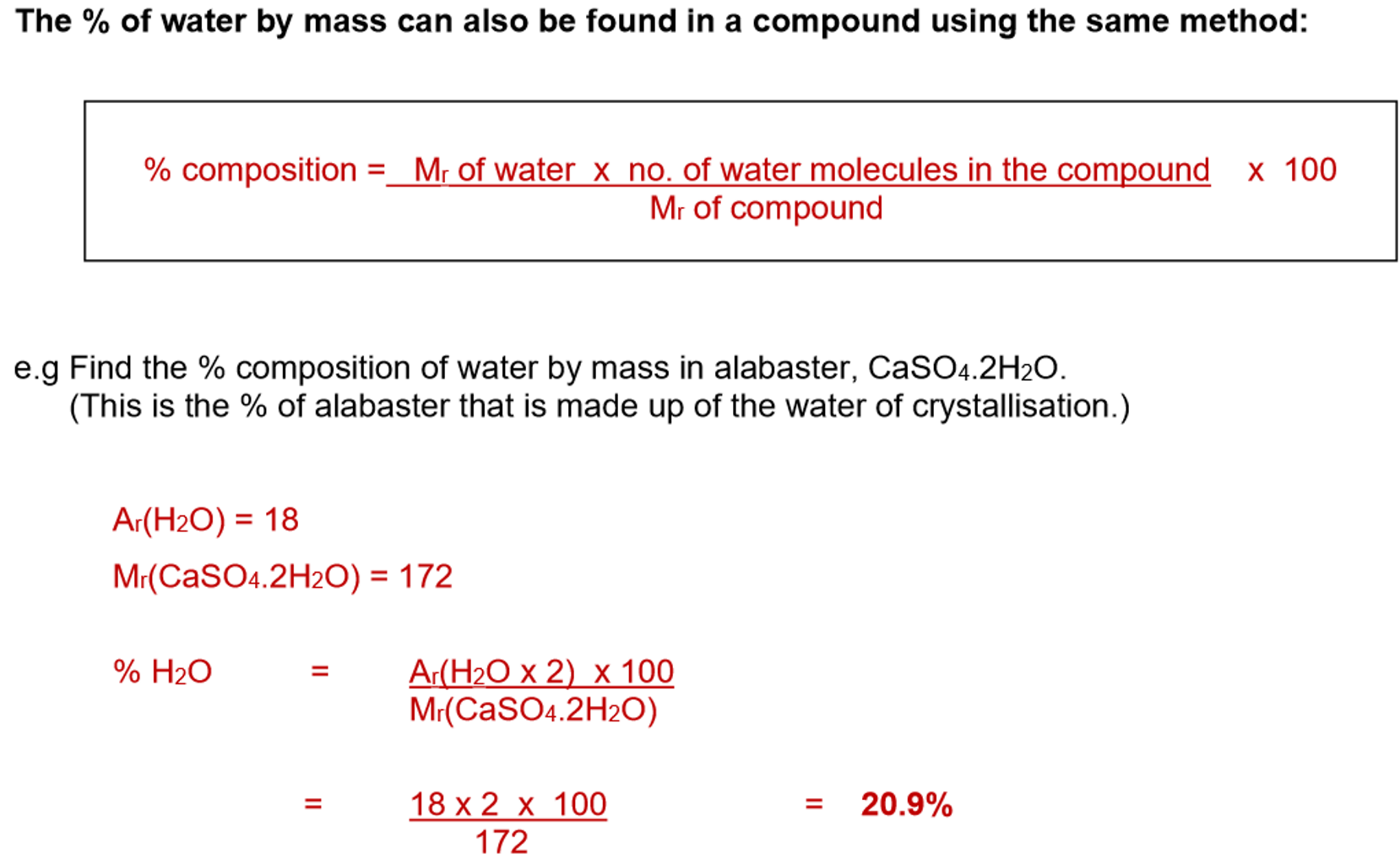
3. Percentage composition formula:
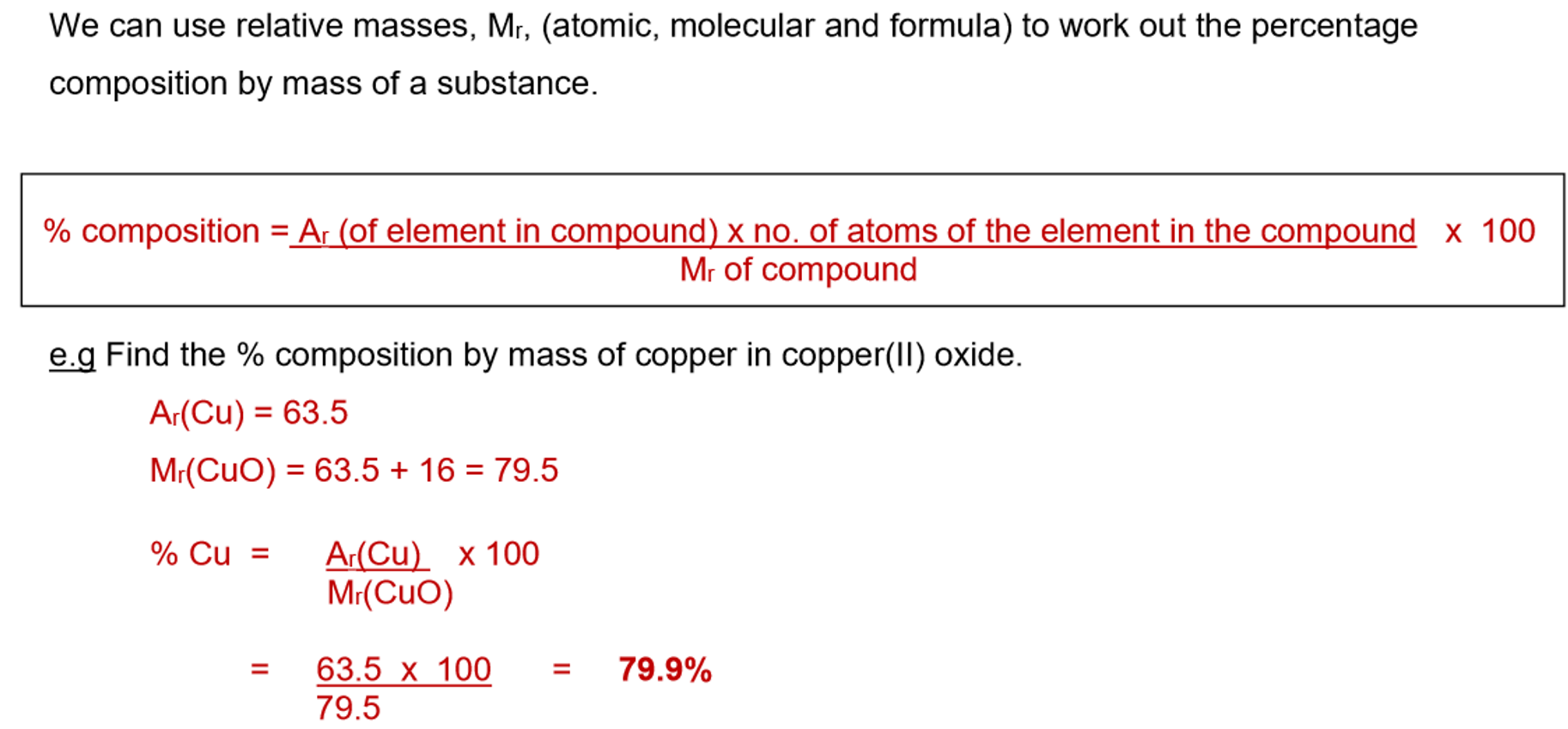
Determining Formula of Metal Oxides by Experiment
Combustion (e.g., Magnesium Oxide, MgO):
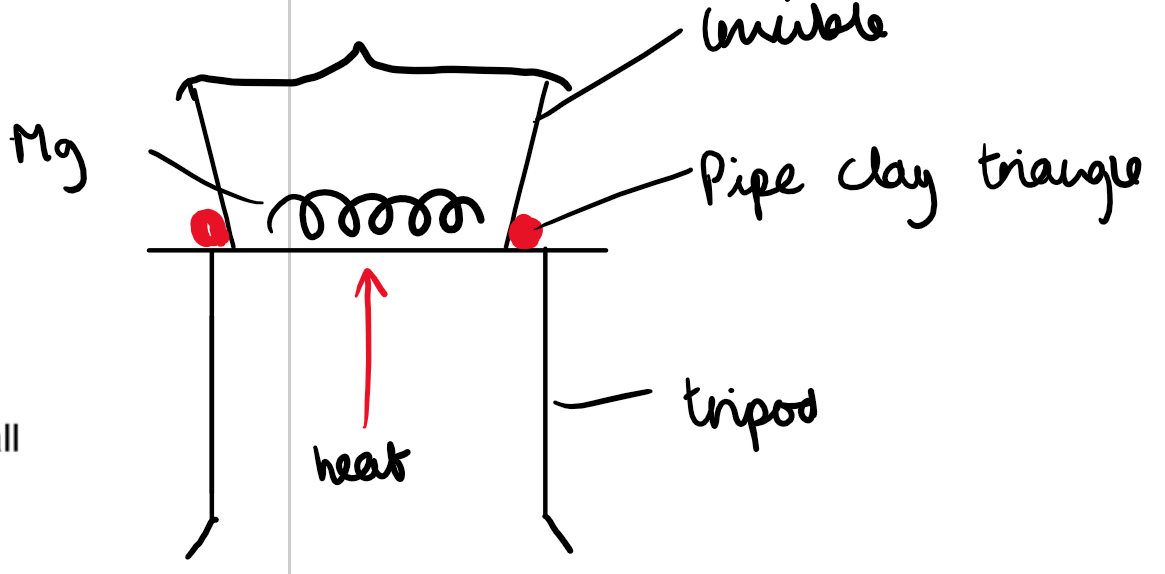
To determine the empirical formula of magnesium oxide:
Weigh a piece of magnesium before burning.
Heat the magnesium in a crucible in the presence of oxygen to form magnesium oxide (MgO).
After the reaction, reweigh the crucible to find the mass of the magnesium oxide.
Use the mass difference (oxygen added) and the initial magnesium mass to calculate the moles of magnesium and oxygen.
Example:
If 2 g of magnesium reacts with oxygen and forms 3.32 g of magnesium oxide, the mass of oxygen is 3.32 g - 2 g = 1.32 g.
Moles of Mg = 2 ÷ 24 = 0.083 mol, Moles of O = 1.32 ÷ 16 = 0.082 mol.
Ratio Mg
≈ 1:1, so the empirical formula is MgO.
Reduction (e.g., Copper(II) Oxide, CuO):
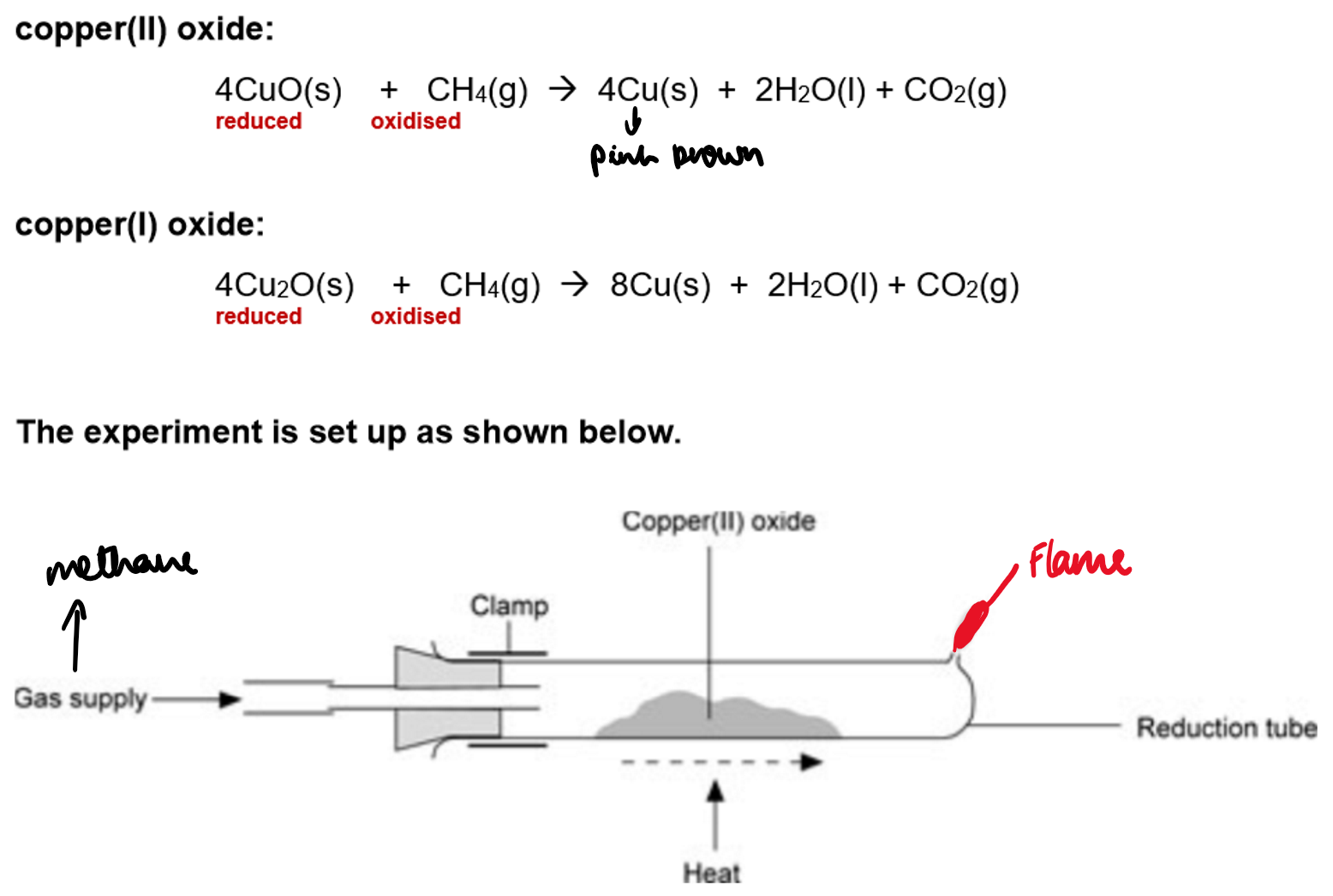
To determine the formula of copper(II) oxide:
Weigh a sample of copper(II) oxide (CuO).
Heat it in a stream of hydrogen gas, which reduces the CuO to copper (Cu).
Weigh the mass of the remaining copper to find the mass of oxygen that has been removed.
Example:
If 2.5 g of CuO is reduced to 2 g of Cu, the mass of oxygen removed is 2.5 g - 2 g = 0.5 g.
Moles of Cu = 2 ÷ 63.5 = 0.0315 mol, Moles of O = 0.5 ÷ 16 = 0.03125 mol.
Ratio Cu
≈ 1:1, so the empirical formula is CuO.
Practical Tips for Formula Determination
Accuracy: Ensure that mass measurements are accurate, especially before and after reactions. Use analytical balances when possible.
Completeness of Reaction: Ensure that the metal has fully reacted (combustion) or been completely reduced. Incomplete reactions may give incorrect results.
Safety Considerations:
Handle heated equipment with care, using tongs and heatproof gloves.
Be cautious when using reactive gases like hydrogen in reduction experiments, and ensure good ventilation.