🌱AP Bio: Unit 0
☆*: .。. o(≧▽≦)o .。.:*☆
Bio 4 Themes:
Here's a breakdown of the four Big Ideas in AP Biology with a focus on the key concepts highlighted in the document:
Big Idea 1: Evolution Drives the Diversity and Unity of Life
Evolutionary Mechanisms
Genetic Variation
Speciation and Extinction
Common Ancestry and Phylogenetics
Origin of Life
Big Idea 2: Biological Systems Utilize Free Energy and Molecular Building Blocks to Grow, Reproduce, and Maintain Dynamic Homeostasis
- Energy Capture and Utilization
- Autotrophic cells using photosynthesis and chemosynthesis to capture free energy.
- Cellular respiration and fermentation as processes for harvesting free energy from sugars.
- Interdependence of photosynthesis and respiration.
- Matter Exchange and Homeostasis
- Exchange of matter with the environment (e.g., water, carbon, oxygen).
- Role of surface-to-volume ratios in resource acquisition and waste elimination.
- Apoptosis in development and differentiation.
- Membrane Function and Selective Permeability
- Cell membranes maintaining internal environments distinct from external surroundings.
- Mechanisms of osmosis, diffusion, and active transport in maintaining homeostasis.
- Compartmentalization in eukaryotic cells for optimal chemical reactions.
- Feedback Mechanisms
- Negative and positive feedback loops in maintaining internal balance.
- Impact of environmental changes on biological responses and resource acquisition.
- Evolution of defense mechanisms against homeostasis disruptions.
- Coordination of Biological Events
- Regulation of developmental, physiological, and behavioral events.
- Role of timing and coordination in increasing fitness and survival.
### Big Idea 3: Living Systems Store, Retrieve, Transmit, and Respond to Information Essential to Life Processes
- Genetic Information and Continuity
- DNA replication ensuring high fidelity transmission of genetic information.
- Role of mutations and errors in introducing heritable variation.
- Impact of nucleotide sequence changes on protein structure and function.
- Gene Expression and Regulation
- Transcription and translation processes controlling cellular metabolism and phenotypes.
- Environmental and developmental signals modulating gene expression.
- Cell signaling pathways influencing gene expression and protein activity.
- Inheritance and Reproduction
- Mitosis and meiosis as mechanisms for genetic continuity and variation.
- Mendelian and non-Mendelian inheritance patterns.
- Ethical and social implications of understanding human genetics.
- Genetic Variation and Evolution
- Importance of genetic variation for species survival and evolution.
- Mechanisms introducing variation in sexually and asexually reproducing organisms.
- Horizontal gene transfer in bacteria and viruses contributing to genetic diversity.
- Information Processing and Behavior
- Role of sensory organs and nervous systems in processing external information.
- Behavioral responses increasing fitness and survival at the individual and population levels.
### Big Idea 4: Biological Systems Interact, and These Systems and Their Interactions Possess Complex Properties
- Systems Interactions and Emergent Properties
- Complex interactions at molecular, cellular, and ecosystem levels.
- Emergent properties resulting from interactions among system components.
- Robustness and flexibility of complex biological systems in responding to environmental changes.
- Molecular and Cellular Interactions
- Influence of molecular subcomponents on polymer properties.
- Coordination among organelles in maintaining cell function and growth.
- Specialization and differentiation driven by gene expression and external stimuli.
- Organismal Interactions
- Coordination among organs and systems ensuring organismal survival.
- Effects of environmental changes on organ responses and overall organism health.
- Ecosystem Dynamics
- Community structure changes in response to environmental conditions.
- Species interactions driving the movement of matter and energy in ecosystems.
- Competition, Cooperation, and Flexibility
- Competition and cooperation among cells and organisms influencing system efficiency.
- Coordination of organs and systems for effective resource use.
- Genetic variation providing flexibility for populations to adapt to environmental changes.
Experimental Design
Hypothesis
Does light color affect plant growth?
Null hypothesis:
Hypothesis that there is NO significant effect, difference, or trend (opposite of the alternative hypothesis)
i.e. Light color does not affect plant growth
Alternative hypothesis:
Hypothesis that there is a SIGNIFICANT effect, difference, or trend
i.e. Light color does affect plant growth
Variables
Independent/Manipulated
The actual thing being changed and tested during the experiment
i.e. Light color
Dependent/Responsive
The response to the independent variable; What will be measured to determine the effect of the independent variable
i.e. plant growth
*Control
`Held constant through the experiment so they do not affect the outcome
i.e. Type of plant, amount of water, type of soil, temperature, light intensity, etc. kept the same
Groups
Experimental- Receives the independent variable to test it
Control- Group that does not receive the independent variable in order to be able to compare the results of the experimental group
Positive: expect a result
i.e. normal full spectrum sunlight
Negative: expect no result—receive no treatment
i.e. No light
Graphing- DRY MIX
Dependent, Manipulated, Responding, Independent
Scatterplots
Qualitative data not manipulated variable or multiple trials
Typically include a trend line
Line
To show change in the dependent variable over time or some continuum
Box and Whisker
Compare variability of 2+ sets of data
Interpreting Results
Error bars
**Represent +/- 2SEM (Standard error of the mean)
If error bars overlap -> not a significant difference between the means
If error bars do not overlap -> significant difference between the means
Writing a conclusion
Claim- statement that responds to the question that was investigated
Evidence- Provide data from your evidence to support your claim
Reasoning- Explain how your data supports your claim
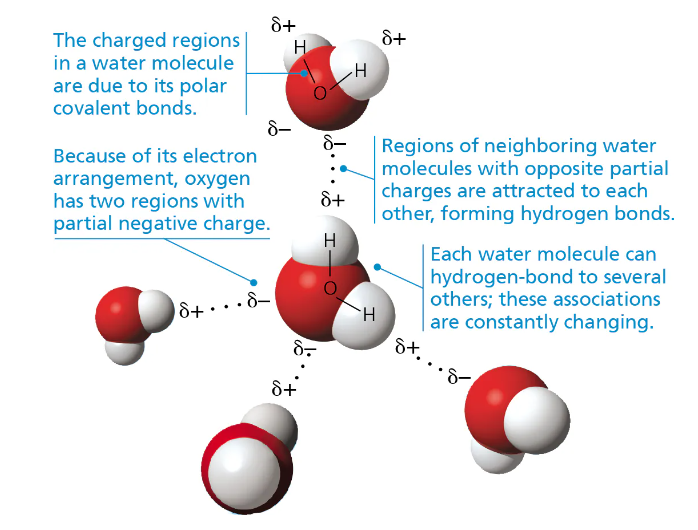
Properties of Water 💧
Causes of Water’s Properties:
Polarity
Caused by the UNEQUAL sharing of electrons in a COVALENT bond between hydrogen/oxygen → unequal distribution of charge in molecules with 2 partial NEGATIVE charges on oxygen + partial POSITIVE charges on hydrogen
2 Types of bonds:
Covalent: Sharing of electrons
Ionic: Transfer of electrons
Hydrogen Bonds:
A strong attractive force between POLAR molecules (polar ± ends attracted)
i.e. Oxygen + Hydrogen; Nitrogen + Hydrogen
Properties of Water:
Cohesion: Water molecules stick to EACH OTHER → transport of water up roots
Adhesion: Water molecules stick to OTHER POLAR surfaces → stick to sides of tubes etc.
High Surface Tension: Difficult to stretch/break the surface of water → insects can glide across water surface
High Specific Heat- Ability to stabilize temperatures b/c requires a large amount of heat stored/lost to change its temperature
Moderates Temperatures: absorbs heat from the warmer air and releases heat to cooler air
High Heat of Vaporization: Water must absorb a high quantity of heat to transform from a liquid to gaseous state → Evaporative cooling: Heat released from surface as water evaporates contributes to temperature stability
Universal Solvent: Dissolves other polar substances (can be ionic)
Water’s Density- Water reaches its greatest density at 4 degrees Celcius—enables ice to float on top of water since water’s solid form is less dense than liquid
pH Level 🧪
Water as a Solvent:
Polar water molecules separate ionic compounds into their constituent ions
Does NOT dissolve nonpolar substances (fats, oil)
MUST be polar, can be ionic but doesn’t have to be to dissolve in water (i.e. sugar)
I. Hydrophilic - “water-loving” POLAR molecules that dissolve in water; polar regions attract water molecules = easily broken apart
II. Hydrophobic - “water-hating” nonpolar molecules that DO NOT dissolve in water b/c not attracted to polar regions of water molcules
Ionization: The formation of ions (charged atoms) by polar molecules in a solvent (dissolves other substances)
In liquid water hydrogen (H+) atom often jumps from the oxygen (O) atom it is COVALENTLY bonded to the oxygen atom it is HYDROGEN bonded
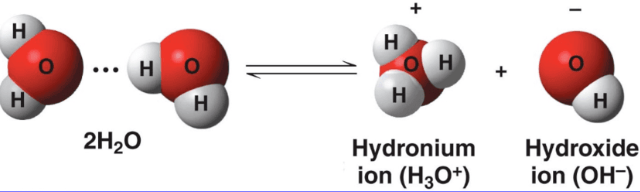
Acids and Bases:
Water serves as BOTH an acid & base
Water dissociates and produces Hydronium (H30+) and Hydroxide (OH-) ions
In pure water, the number of hydrogen (H+) ions equal the number of hydroxide (OH-) ions
Both very REACTIVE can drastically impact cells—must control their concentrations
Acids- PRODUCES/donates (H+) ions/protons when dissolved
Acidic when (H+) > (OH-)
Bases- ACEEPTS (H+) ions/protons
Basic when (H+) < (OH-)
pH scale- measures the acidity/concentration of (H+) ions in a substance ranging from (0-14); LOGARITHMIC
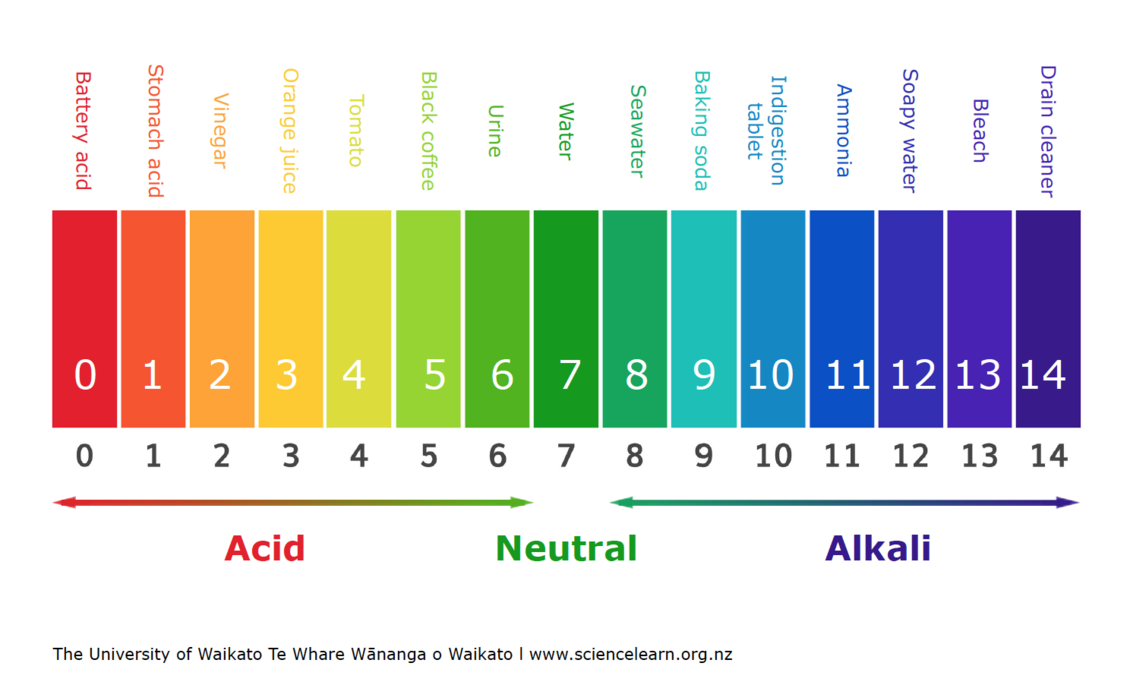
Lower the pH = MORE acidic/(H+) ions; pH of 7 = neutral; Below 7 = acidic; Above 7 = basic
Each pH unit represents 10x difference; Ex. pH 3 1000x as acidic as pH 6 (10³)
*pH = -log10[H+]; Ex. H+ concentration = 10-7 -log[10^-7] = -(-7) = 7 OR positive # of the exponent
*[OH-] and [H+] have a PRODUCT of 1.0 x 10^-14M; find one given another by dividing 10^-14 by the given/subtracting exponents
*pOH - OPPOSITE of pH scale—lower = basic, 7 = neutral, higher = acidic
Living Organisms and pH
Organisms attempt to maintain neutral pH level b/c enzymes can be denatured in pH changes
Buffers: weak acids/bases that are (H+)/(OH-) ion ACCEPTORS; living cells produce buffers to absorb excess (H+)/(OH-) and maintain neutral pH level
Ex. (H2CO3)Carbonic Acid ←→ (HCO3-)Bicarbonate + (H+) moves Right when too BASIC/Left when too ACIDIC
Tricky Practice Problems:
What is the pOH of a solution with a pH of 2
10^-14/10^-2 = 10^-12 -log[10^-12] = 12
OR 14 - 2 = 12
A liquid with a pH of 4 is how many times more concentrated in H+ than a liquid with a pH of 6?
6 - 4 = 2; 10² = 100
The [H+] of a juice is 0.00010 M, find the pH
Count zeroes after 1; 1.0 × 10^-4 = 4
The greater the pOH the SMALLER the concentration of:
OH- remember pH/pOH scales OPPOSITE higher the pH/pOH = lesser the concentration of respective substances
Statistics and Standard Deviation 📊
Standard Deviation (x1- mean)² + (x2-mean)²…etc
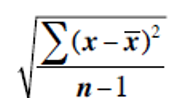
Standard Error of the Mean
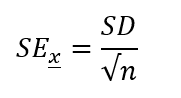
Variance