Introduction to Separation Method 2.docx
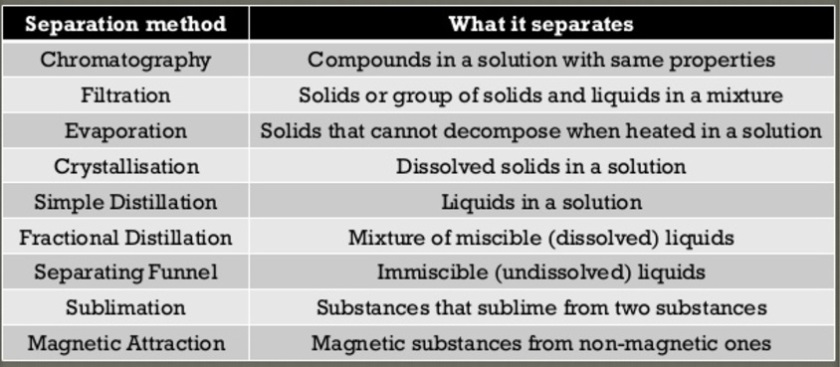
Introduction to Separation Techniques
Learning Objectives:
Identify and explain the principles behind a particular separation
technique that is used in daily life and in industry.Identify an appropriate separation technique to separate a mixture based
on the physical properties of the components of the mixture. These
properties include solubility, density, melting and boiling points, thermal
stability, magnetic properties and particle size.
Separation techniques are used to separate mixtures into its constituent elements and/or compounds. Recall that a mixture is contains elements and/or compounds which are not chemically combined together.
By separating the constituents of the mixtures, we are able to find out the properties of the known/unknown substances from mixtures and possibly use them for the production of useful substances such as medicines.
Depending on the physical and chemical properties of the substances in the mixture, we can choose the most appropriate separation technique to isolate them from the mixture.
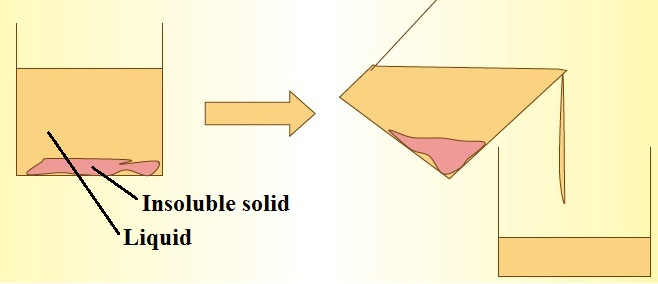
Decanting: A crude way of separating insoluble solids from liquids, as the liquid is poured away and collected in another container. Note that the insoluble solid should be able to settle down on standing and this method is not effective for obtaining clear liquid from the mixture especially when the insoluble solid is very fine and light.
Filtration: Separation of solids or groups of solids from the liquid in a mixture, using a medium through which the liquid can pass.
The medium which we are using over here is the filter paper. The filter paper is folded and placed onto the filter funnel.
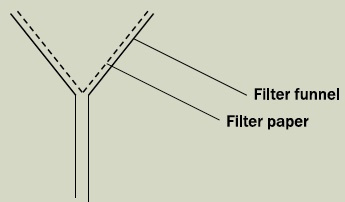
The liquid-solid mixture is poured onto the filter paper. Using a filter paper with pores of a smaller size than the solid particles (and is larger than the size of the liquid molecules), the liquid (or solvent) should pass through the filter paper, and is collected by a collection container placed at the bottom of the filter funnel.
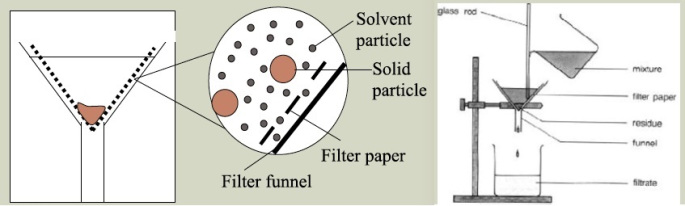
The liquid that passes through the filter paper is called the filtrate while the solid left on the filter paper is called the residue.
Evaporation: In the case which we do not need to collect the solvent. The solvent is boiled off and escape into the air while the solute is left behind in the holding container. Note that this method is not suitable for use on solutes which can decomposed by heating (e.g. Copper II sulfate).
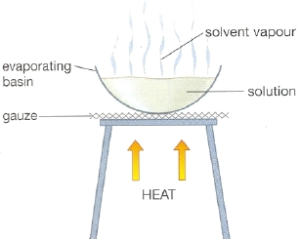
Crystallisation: Used to separate a dissolved heat-liable (will decompose upon heating and hence can sublime) solid (solute) from a solution.
You will need a saturated solution to being with. A saturated solution is a solution that contains the maximum amount of solute dissolved in a given volume of solvent at a particular temperature. Do not mix this up with a concentrated solution, which is a solution that contains lots of solute dissolved in it. The amount of solute in a concentrated solution may/may not be the maximum amount which can be dissolved in the solution.
First, you will need to heat to evaporate off most of the solvent from a solution to make a hot and nearly saturated solution. Else, if you already have a saturated solution, heat it up slightly such that the solution becomes hot.
After which, allow the hot solution to coll naturally. The solubility of the solute decreases as the solution is cooled, and the excess solute which can no longer be dissolved in the saturated solution crystallizes out of the solution. The crystals which are formed can be separated from the remaining solution by filtration.
Seeding: Using a small crystal of salt (the “seed”) to collect the solid solute crystals in a saturated solution. No heat is required but will take a long time.
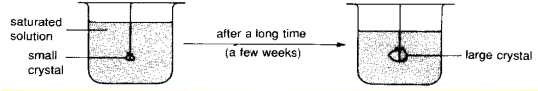
As the saturated solution continues to evaporate overtime, there is an increasing amount of solute which will crystallise out from the solution. These solid solute crystals will attach itself to the “seed”, resulting in a grow in size of the “seed” overtime. The enlarged “seed” can then be removed from the solution at the end of the procedure (or when it is large enough for use in other applications).
Simple Distillation: To separate and collect solvent from a solution of solutes, or in a mixture of two different liquids (with different boiling points), with the use of heat.
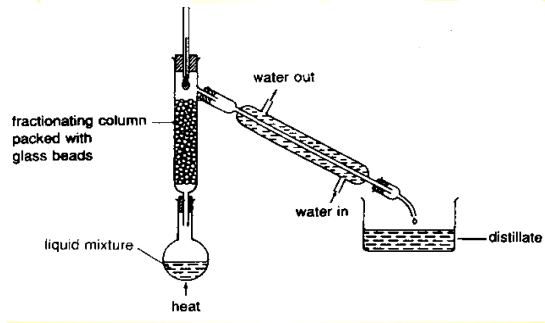
The logic behind how simple distillation works is actually the same as that of evaporation. The only difference is that a closed neck container (distillation flask) is used to hold the mixture to be heated, with a opening/tube by the side (of the container) connected to a condenser. The setup for simple distillation should look something like this:
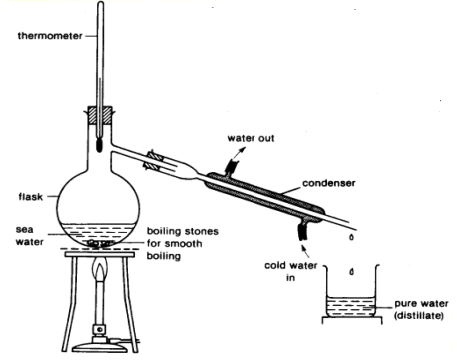
As the sea water mixture is heated, water boil and changes into water vapour gas. Since hot air rises and cold air sinks, the hot water vapour moves to the top of the flask and passes into the condenser.
The tubes on the condenser are attached to a water source, with the water flowing in through the lower end and flowing out through the higher end of the condenser. This creates a cooler surface for the hot water vapour to condense on. As the condenser is tilted downwards, towards the collecting container at the end of the setup, the condensed water flows and drips into the collecting container.
Fractional Distillation: Used to separate miscible liquids with different but very close boiling point. This method is more efficient than simple distillation.
A fractionating column is introduced between the distillation flask and the condenser. The upper portion of the column, which is closer to the condenser, is cooler than the lower portion and hence, only gases with the same temperature as the upper portion are allowed to pass on to the condenser. On the other hand, the gases with higher boiling points will condense and flow back to the bottom into the distillation flask, and is heated into a gas again. At the end, liquid with the lowest boiling point will be the first to boil and hence the first to be distilled out and collected.
Sublimation: To separate a mixture of solids containing one which sublimes and one (or more than one) which does not, by heating the mixture.
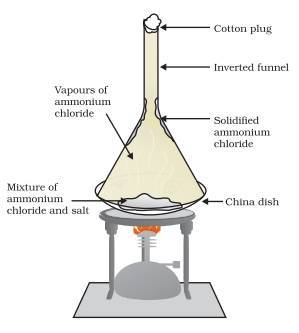
An cotton-stoppered inverted funnel is placed over the mixture. When the mixture is heated, the heat-liable solid sublime and turn into a gas, and travel to the top of the inverted funnel. Once the hot gas touches the cooler funnel, it solidifies back into a solid. The solid can then be scrapped off and collected in another container from the funnel.
Magnetization or Magnetic Attraction: This method involves the separation of magnetic substances from non-magnetic substances by means of a magnet.
Paper Chromatography: Used to separate a mixture of solutes (or liquid) with different solubility and degree of adsorption. This method uses a porous or absorbant medium (e.g. paper or jel) and a solvent which can move over the material. This method is commonly used for separating a mixture of dyes in ink or different types of sugars (e.g. glucose, fructose, sucrose).
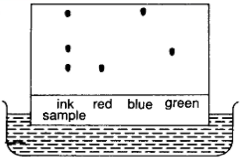
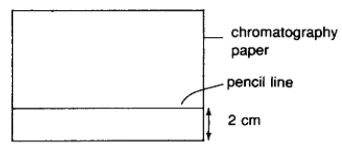
In particular, to carry out paper chromatography, get a rectangular piece of filter paper and draw a pencil line 2 cm away from the bottle edge of the paper. Note that pen cannot be used to draw the line here as its ink mixture will be resolved and there will be no reference line left at the end of the experiment.
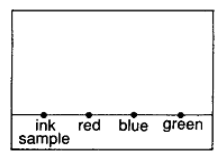
Place a drop of sample ink or mixture on the line. Let the sample dry before placing more sample on the same spot. Place the other known components (e.g. red, blue and green dye if we are separation an ink sample) on the line, with a distance away from the first ink sample, for comparison.
Place the chromatography paper on a suitable solvent (e.g. ethanol). The solvent will “run” up the chromatography paper after some time.
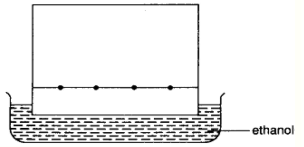
As the solvent travels up the paper, the dyes are dissolved in the solvent. Since some dyes are more soluble, they travel up the paper faster than the rest. The less soluble dyes are absorbed more strongly on the paper near the pencil line. This means that identical dyes will travel the same distance along the length of the paper.
When the solvent reaches near the top of the paper, remove the paper and mark the location where the solvent stops running. By comparing the ink sample with the colour dyes, we know the composition of the ink sample. For this case, we can conclude that the ink sample contain red, blue and green dyes.