Review Final Exam May 1
Gluconeogenesis and Glycolysis Overview
Gluconeogenesis
Definition: The metabolic process of synthesizing glucose from non-carbohydrate sources, primarily occurring in the liver and to some extent in the kidney.
Importance: Essential for maintaining blood glucose levels, especially during fasting, prolonged exercise, or carbohydrate restriction.
Glycogenolysis
Definition: The biochemical breakdown of glycogen (stored glucose) into glucose molecules.
Function: Provides a rapid source of glucose for energy needs, especially critical when blood sugar levels drop.
Regulation: Controlled by hormonal signals such as glucagon and epinephrine that trigger glycogen breakdown to release glucose.
Key Intermediates
Lactate:
Produced during anaerobic metabolism, particularly in exercising muscles.
Can be recycled in the liver to form glucose through the Cori cycle, allowing for energy production in low oxygen conditions during intense activity.
carbon substrate
assists in pyruvate metabolism
Amino Acids
carbon substrate
assists in pyruvate metabolism
assists in TCA cycle
Pyruvate:
A central metabolite that serves as an intersection between numerous metabolic pathways including glycolysis, lactate metabolism, and amino acid breakdown.
Can be converted to acetyl-CoA for entry into the TCA cycle when oxygen is available, or it may enter gluconeogenesis to produce glucose when energy is needed.
Ketone Bodies:
Produced in the liver during prolonged fasting or carbohydrate restriction from fatty acid metabolism.
Serve as an alternative energy source for many tissues, including the brain, allowing the body to utilize fat stores when glucose is scarce.
Alanine:
An amino acid that can be converted into pyruvate through transamination reactions.
Represents the role of protein catabolism in gluconeogenesis, especially during fasting or muscle wasting states.
Glycerol:
Derived from the breakdown of triglycerides in fat cells.
Serves as a substrate for gluconeogenesis, connecting lipid metabolism with carbohydrate production.
Role of Key Enzymes
Lactate Dehydrogenase (LDH):
Enzyme that catalyzes the conversion of lactate to pyruvate.
Important for recycling lactate during exercise to regenerate glucose through gluconeogenesis.
Glucose-6-Phosphatase:
Enzyme that converts glucose-6-phosphate into free glucose.
Enables the release of glucose into the bloodstream, crucial for maintaining blood glucose homeostasis, particularly during fasting.
Fructose-1,6-Bisphosphatase:
A key regulatory enzyme in gluconeogenesis that catalyzes the conversion of fructose-1,6-bisphosphate to fructose-6-phosphate, effectively inhibiting glycolysis.
Plays a crucial role in maintaining the balance of glucose production and utilization, opposing phosphofructokinase-1 (PFK-1) in glycolysis.
Pyruvate & the Pyruvate Dehydrogenase Complex (PDC)
Inhibition of PDC:
Its inhibition is crucial during gluconeogenesis to prevent the conversion of pyruvate to acetyl-CoA, redirecting pyruvate towards glucose formation.
Pyruvate Kinase:
Converts phosphoenolpyruvate (PEP) to pyruvate in glycolysis but is bypassed in gluconeogenesis by the use of PEP carboxykinase (PEPCK).
NADH/NAD+ Ratio:
A high ratio favors gluconeogenesis, indicating a sufficient energy state. Low ratios favor glycolysis, demonstrating how cellular energy state influences metabolic pathways.
Inhibition of Pyruvate Dehydrogenase Complex (PDC)
Overview:
The Pyruvate Dehydrogenase Complex (PDC) is essential for converting pyruvate into acetyl-CoA, linking glycolysis to the TCA cycle. Its inhibition plays a vital role in regulating metabolic pathways, especially during gluconeogenesis.
Importance of Inhibition:
Redirecting Pyruvate:
Inhibition of PDC during gluconeogenesis is crucial as it prevents the conversion of pyruvate to acetyl-CoA, redirecting pyruvate towards glucose formation instead.
Energy Conservation:
During fasting or energy-demanding situations, inhibiting PDC conserves energy for gluconeogenic processes, ensuring glucose production instead of entering the TCA cycle.
Mechanisms of Inhibition:
Inhibition can occur in response to elevated levels of products like acetyl-CoA and NADH, signaling sufficient energy status in the cell and favoring gluconeogenesis over further oxidative pathways.
High levels of acetyl-CoA and NADH from FA oxidation act to inhibit PDC
Conclusion:
Inhibiting the Pyruvate Dehydrogenase Complex is a critical regulatory step for efficiently producing glucose from non-carbohydrate sources, particularly when glucose is needed during periods of energy deficit or fasting.
Biotin in Biochemical Reactions
Biotin:
This essential vitamin acts as a cofactor for pyruvate carboxylase, facilitating the conversion of pyruvate to oxaloacetate—a critical step for gluconeogenesis.
Biotin's role in the conversion process involves the transfer of a CO2 molecule to pyruvate, demonstrating its importance in carbon metabolism and the initiation of gluconeogenesis.
Biotin in Pyruvate carboxylase reaction
Biotin is a cofactor covalently attached to the enzyme through an amide linkage to the zeta amino group of a Lys residue
biotinyl-enzyme
The reaction occurs in two phases, which occur at two different sites in the enzyme
at catalytic site 1, bicarbonate ion is converted to CO2 at the expense of ATP
forms Carboxyphosphate
CO2 reacts with biotin
creates carboxy-biotinyl-enzyme
Biotin-Lysine: a long arm that carries CO2 of carboxy-biotinyl-enzyme to catalytic site 2
CO2 is released and reacts with pyruvate
creates oxaloacetate
Phosphoenolpyruvate Carboxykinase (PEPCK)
Function:
Converts oxaloacetate into phosphoenolpyruvate (PEP) utilizing GTP as an energy source, facilitating an essential step in gluconeogenesis.
Substrate Specificity:
Specifically expressed in liver and kidney tissues, highlighting its critical function in regulating gluconeogenic capacity and the energy balance in these organs especially during fasting.
Production of PEPCK
Arg finger polarizes carbonyl O of OAA
Arg finger organizes Gamma Pi of GTP/ATP
C3-C4 → C2=C3 enol pyruvate
C2 oxyanion formation
C3 releases CO2
C2 oxyanion attacks gamma PI
PEP is produced
Mechanism to prevent the Futile Cycle During Glycolysis and Gluconeogenesis
Regulatory Enzymes:
The pathways of glycolysis and gluconeogenesis are controlled by key regulatory enzymes that are oppositely regulated. For example:
Fructose-1,6-Bisphosphatase (gluconeogenesis) is activated when energy is abundant and glucose is needed, while it is inhibited by fructose-2,6-bisphosphate and AMP, which promote glycolysis.
Phosphofructokinase-1 (PFK-1) (glycolysis) is activated by AMP and fructose-2,6-bisphosphate, signaling the need for glucose breakdown for energy.
Hormonal Regulation:
Hormones play a crucial role in switching between glycolysis and gluconeogenesis depending on the body’s energy needs:
Insulin promotes glycolysis and inhibits gluconeogenesis to facilitate glucose uptake and usage during fed states.
Glucagon stimulates gluconeogenesis and glycogenolysis, raising blood glucose levels during fasting.
Substrate Availability:
The availability of substrates and energy state also influences which pathway predominates:
High levels of ATP and citrate favor gluconeogenesis, while low energy states (high ADP/AMP) favor glycolysis.
Conclusion:
By employing these mechanisms, the body effectively prevents the futile cycle, ensuring that either glycolysis or gluconeogenesis is active based on the metabolic needs, thus optimizing energy utilization and maintaining glucose homeostasis.
Regulation of Glycolysis vs. Gluconeogenesis
Fructose-2,6-Bisphosphate:
A crucial regulatory metabolite that governs the balance of glycolysis and gluconeogenesis; elevated levels signal the need for glycolysis, while low levels favor gluconeogenesis, underscoring the importance of metabolic control.
Hormonal Regulation:
Insulin promotes glycolysis and inhibits gluconeogenesis, facilitating glucose uptake and utilization. Glucagon, on the other hand, stimulates gluconeogenesis and glycogenolysis, raising blood glucose levels in response to hypoglycemia.
Process of glucagon in reducing Fructose 2,6-Biphosphate concentration and enhancing gluconeogenesis in the liver
High glucagon/insulin ratio causes elevated cAMP and increased levels of active protein kinase A
increased protein kinase A activity favors the phosphorylated form of bifunction PFK-2/FBP-2
phosphorylation of PFK-2 domain inactivates it allowing the FBP-2 domain to be active
decreased levels of fructose 2,6-biphosphate decreases the inhibition of FBP-1, which leads to an increased rate of gluconeogenesis
The pyruvate dehydrogenase complex and the Krebs (TCA) cycle
pyruvate dehydrogenase complex
Substrate:
Pyruvate, derived from glycolysis, enters the PDC.
Decarboxylation:
One carbon from pyruvate is removed as CO2, converting the 3-carbon pyruvate into a 2-carbon acetyl group.
Formation of Acetyl-CoA:
The remaining 2-carbon acetyl group is joined with Coenzyme A (CoA) to form Acetyl-CoA.
This reaction also generates NADH from NAD+.
The overall reaction:
Pyruvate + CoA + NAD+ → Acetyl-CoA + CO2 + NADH
Regulation of PDC:
PDC is regulated by the availability of substrates as well as feedback from its products (Acetyl-CoA, NADH), and through covalent modification (phosphorylation).
Krebs Cycle (TCA Cycle)
Entry of Acetyl-CoA:
Acetyl-CoA combines with oxaloacetate to form citrate (6 carbons) in the first step of the cycle.
Enzyme: Citrate synthase.
Conversion to Isocitrate:
Citrate is rearranged to isocitrate.
Enzyme: Aconitase.
Decarboxylation to Alpha-Ketoglutarate:
Isocitrate is oxidized to alpha-ketoglutarate, generating NADH and releasing CO2.
Enzyme: Isocitrate dehydrogenase.
Decarboxylation to Succinyl-CoA:
Alpha-ketoglutarate is further oxidized to succinyl-CoA, producing another NADH and releasing CO2.
Enzyme: Alpha-ketoglutarate dehydrogenase.
Conversion to Succinate:
Succinyl-CoA is converted to succinate, generating GTP or ATP (depending on the cell type).
Enzyme: Succinyl-CoA synthetase.
Oxidation to Fumarate:
Succinate is oxidized to fumarate, producing FADH2 in the process.
Enzyme: Succinate dehydrogenase.
Hydration to Malate:
Fumarate is hydrated to malate.
Enzyme: Fumarase.
Oxidation to Oxaloacetate:
Malate is oxidized to regenerate oxaloacetate, producing another NADH.
Enzyme: Malate dehydrogenase.
Cycle Continuation:
The regenerated oxaloacetate can combine with another Acetyl-CoA, thus continuing the cycle.
summary
The PDC and Krebs Cycle are critical for converting pyruvate into energy-rich molecules (NADH, FADH2, GTP/ATP) which are essential for subsequent ATP production through oxidative phosphorylation. Each step in these cycles is tightly regulated to maintain metabolic control within the cell.
PDC uses 3 catalytic co-enzymes (prosthetic groups)
Pyruvate dehydrogenase, E1
Dihydrolipoyl transacetylase, E2
Dihydrolipoyl dehydrogenase, E3
Process
Decarboxylation of pyruvate by TPP on Pyruvate Dehydrogenase (E1). Hydroexyethyl TPP is form as an intermediate
regulation of the PDH complex
pyruvate is directed towards gluconeogenesis because of high acetyl-CoA and NADH from FA oxidation act to inhibit the PDC
PDC is inactive during gluconeogenesis
ATP
Kinase
NADH+
Acetyl CoA+
Pyruvate-
ADP-
ADP
Ca2+ is a strong activator of the phosphatase in the muscle
PDC is active during glycolysis → TCA
Isomerization of Citrate to Isocitrate
Overview:
The isomerization of citrate to isocitrate is a key step in the Krebs (TCA) cycle. This transformation is essential for the subsequent reactions in the cycle leading to energy production.
Mechanism:
Enzyme Catalysis:
The enzyme responsible for this conversion is aconitase.
Initial Reaction - Citrate to cis-Aconitate:
The reaction begins with citrate, a 6-carbon molecule, produced from the condensation of acetyl-CoA and oxaloacetate.
Aconitase catalyzes the removal of one water molecule from citrate, forming an intermediate called cis-aconitate.
Rehydration - cis-Aconitate to Isocitrate:
The enzyme aconitase then adds back a water molecule, facilitating the rearrangement of atoms within the molecule.
This leads to the formation of isocitrate, another 6-carbon molecule that has a different structural arrangement compared to citrate.
Summary of the Reaction:
Citrate ⇌ cis-Aconitate ⇌ Isocitrate
Importance in the Krebs Cycle:
The conversion of citrate to isocitrate is crucial as it prepares the substrate for further oxidation steps within the Krebs cycle, contributing to the overall metabolic pathway that generates ATP and reduces equivalents (NADH and FADH2) for oxidative phosphorylation.
Oxidative-decarboxylation of isocitrate into a-ketoglutarate
steps
isocitrate (a secondary alcohol) is oxidized by hydride transfer to NAD+ or NADP+ (depending on the isocitrate dehydrogenase isozyme)
isocitrate dehydrogenase
makes oxalosuccinate (a ketone)
decarboxylation is facilitated by electron withdrawal by bound Mn2+
CO2
makes an enolate
rearrangement of the enol intermediate generates a-ketoglutarate
makes a-ketoglutarate
notes
isocitrate-dehydrogenase
irreversible oxidative decarboxylation of isocitrate
lose 2 e-, makes first NADH and first release of CO2
one of the rate-limiting steps of the TCA cycle
allosterically activated by ADP (a low energy signal) and Ca2+
allosterically inhibited by ATP and NADH (levels are elevated when energy abundant)
increasing NAD+/NADH speeds up the cycle and vice versa
regulation of the TCA cycle
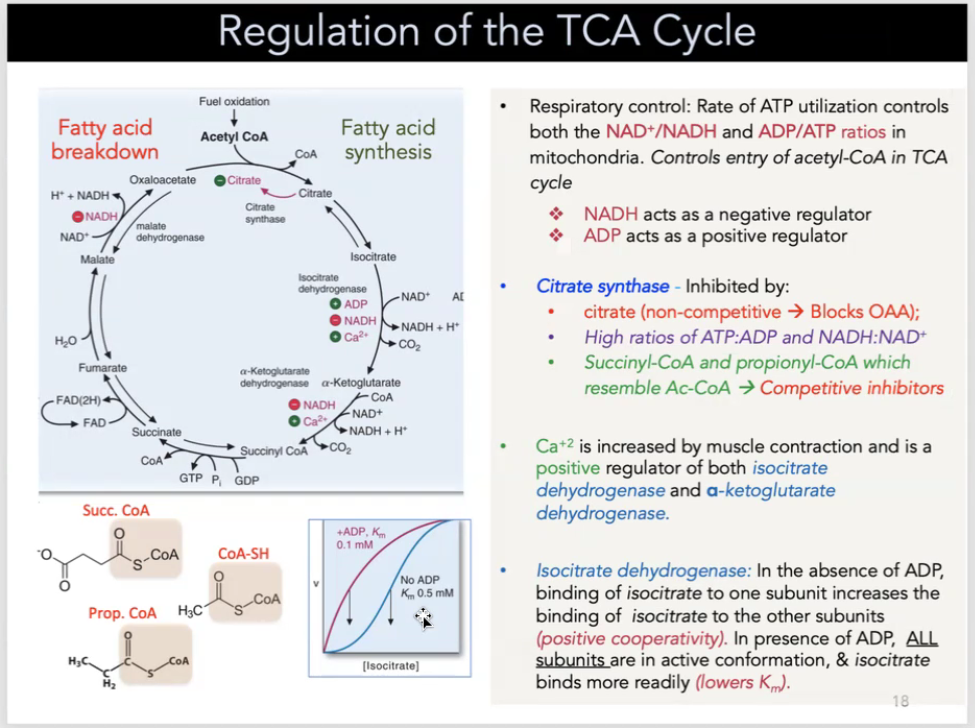
respiratory control: rate of ATP utilization controls both the NAD+/NADH and ADP/ATP ratios in mitochondria—controls entry of acetyl-CoA in TCA cycle
NADH acts as a positive regulator
ADP acts a a negative regulator
Citrate synthase is inhibited by:
citrate (non-competitive → blocks OAA)
high ratios of ATP:ADP and NADH:NAD+
Succincyl-CoA and propionyl-CoA which resemble Ac-CoA → competitive inhibitors
Ca2+ is increased by muscle contraction and is a positive regulator of both isocitrate dehydrogenase and a-ketoglutarate dehydrogenase
isocitrate dehydrogenase: in the absence of ADP, binding of isocitrate to one subunit increases the binding of isocitrate to other subunits (positive cooperativity). In the presence of ADP, ALL SUBUNITS are in active conformation and isocitrate binds more readily (lowers Km)
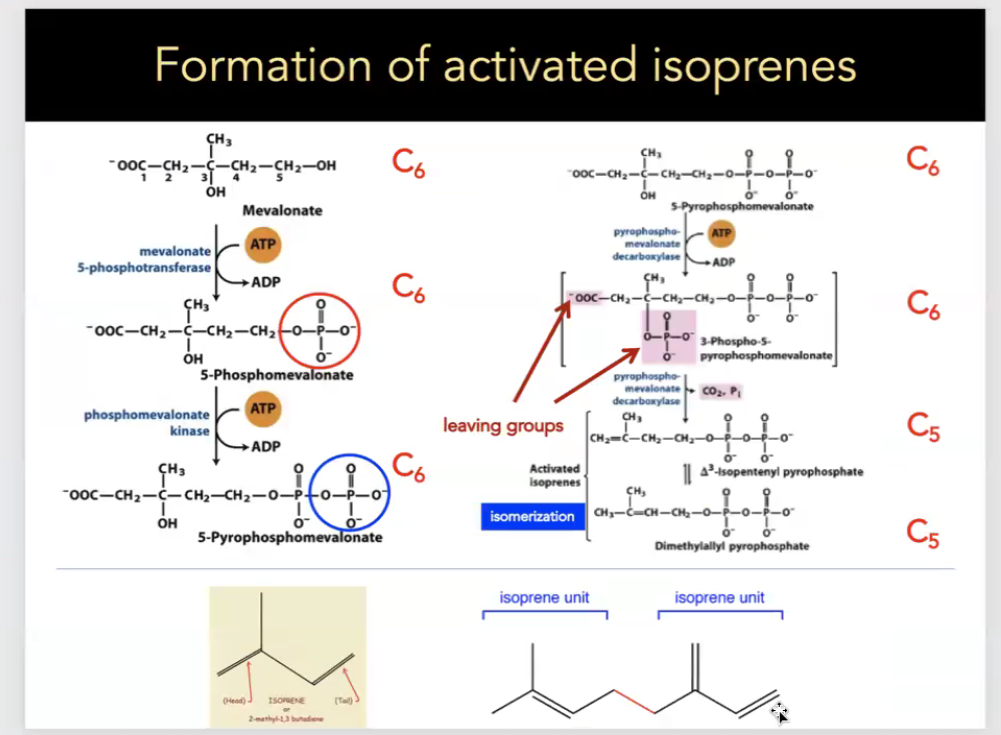
cholesterol synthesis
all C-atoms of cholesterol come from acetate (as acetyl CoA) and reducing equivalents come from NADPH
energy to drive cholesterol synthesis comes from acetyl CoA and ATP hydrolysis
synthesis occurs in the cytoplasm and on the ER
important to note
first two reactions form HMG CoA from acetyl CoA
HMG CoA reductase is the rate limiting (regulated) step of this pathway, resulting in the formation of mevalonic acid from HMG CoA and 2 NADPH
formation of activated isoprenes
Fatty Acid Metabolism
Beta-oxidation:
Fatty acids undergo beta-oxidation to generate acetyl-CoA, which then enters the TCA cycle to produce ATP.
This process illustrates the utilization of fat as an alternate energy source during fasting or prolonged exercise, as they provide substantial energy compared to carbohydrates.
Role of Glycerol:
The conversion of glycerol into glucose via gluconeogenesis highlights the vital link between lipid and carbohydrate metabolism, emphasizing the body's ability to mobilize energy stores effectively.
Carnitine:
An essential molecule that facilitates the transport of long-chain fatty acids into mitochondria for beta-oxidation.
Deficiency can lead to impaired fatty acid oxidation and energy deficits, showcasing its important role in fatty acid metabolism.
Ethanol Metabolism
Ethanol Metabolism:
Occurs primarily in the liver where ethanol is converted to acetaldehyde by Alcohol Dehydrogenase (ADH), which subsequently is converted to acetate by aldehyde dehydrogenase.
This metabolic pathway plays a role in modulating energy metabolism, especially under conditions of excessive alcohol intake.
CYP2E1:
An enzyme involved in the oxidative pathway of ethanol metabolism, which can also contribute to the generation of reactive oxygen species (ROS), thereby leading to alcohol-related liver damage.
Questions on Metabolic Pathways
Understanding Regulation:
Enquires and in-depth analysis about how various enzyme functions interplay within gluconeogenesis and glycolysis highlight the complexities of metabolic control across different states of energy needs.
Enzyme Activities:
Studying specific enzymatic reactions in these pathways offers critical insights into metabolic states and how such changes can affect overall homeostasis.
Practical Applications:
Investigating genetic defects in any of these metabolic pathways underscores their clinical significance, as such issues can lead to metabolic disorders, demonstrating the relevance of each pathway in human health and disease.
Gluconeogenesis Pathway Overview
Location: Primarily occurs in the liver and to some extent in the kidneys.
Starting Molecules: Non-carbohydrate sources like lactate, pyruvate, alanine, and glycerol serve as substrates.
Key Steps:
Conversion of Pyruvate to Phosphoenolpyruvate (PEP):
Pyruvate is converted to oxaloacetate by pyruvate carboxylase (requires ATP and biotin as a cofactor).
Oxaloacetate is then converted to PEP by phosphoenolpyruvate carboxykinase (PEPCK), utilizing GTP.
Reversal of Glycolysis:
Several steps of glycolysis are simply reversed in gluconeogenesis (e.g., conversion of fructose-1,6-bisphosphate to fructose-6-phosphate via fructose-1,6-bisphosphatase).
Final Step - Conversion to Glucose:
Glucose-6-phosphate is converted to glucose by glucose-6-phosphatase, which facilitates the release of glucose into the bloodstream.
Energy Requirement: Gluconeogenesis requires ATP and GTP, demonstrating the energy investment to synthesize glucose from non-carbohydrate precursors.
Regulation:
Hormonal control plays a significant role; insulin inhibits gluconeogenesis while glucagon stimulates it, helping maintain blood glucose levels especially during fasting conditions.
Key Enzymes:
Pyruvate Carboxylase: Converts pyruvate to oxaloacetate.
PEPCK: Converts oxaloacetate to phosphoenolpyruvate.
Fructose-1,6-bisphosphatase: Regulates conversion from fructose-1,6-bisphosphate to fructose-6-phosphate.
Glucose-6-Phosphatase: Transforms glucose-6-phosphate into free glucose for energy use or storage.
Glycogenolysis Overview
Definition:
The biochemical breakdown of glycogen (stored glucose) into glucose molecules.
Function:
Provides a rapid source of glucose for energy needs, especially critical when blood sugar levels drop.
Regulation:
Controlled by hormonal signals such as glucagon and epinephrine that trigger glycogen breakdown to release glucose.
Key Steps in Glycogenolysis:
Phosphorylation of Glycogen:
Glycogen phosphorylase catalyzes the conversion of glycogen to glucose-1-phosphate. This reaction involves the addition of a phosphate group to glycogen.
Conversion to Glucose-6-Phosphate:
Glucose-1-phosphate is then converted to glucose-6-phosphate by phosphoglucomutase.
Release of Free Glucose:
In the liver, glucose-6-phosphate can be converted to free glucose by glucose-6-phosphatase, allowing it to enter the bloodstream and raise blood glucose levels.
Importance:
Glycogenolysis is crucial for maintaining energy levels during periods of fasting or intense physical activity, ensuring that the body has access to glucose when needed.