13.1 to 13.4 Water
13.1 Water chemical tests, treatment and uses
Chemical tests for water
- Cobalt(II) chloride turns blue to pink on the addition of water. This test is usually done using cobalt chloride paper
- The equation is:
- CoCl2 (s) + 6H2O (l) → CoCl2.6H2O (s)
- Anhydrous copper(II) sulfate turns white to blue on the addition of water
- The equation is:
- CuSO4 (s) + 5H2O (l) → CuSO4.5H2O (s)
Water supply
- Water is taken from rivers, reservoirs or underground water sources (groundwater)
- A rock that stores water is known as an aquifer
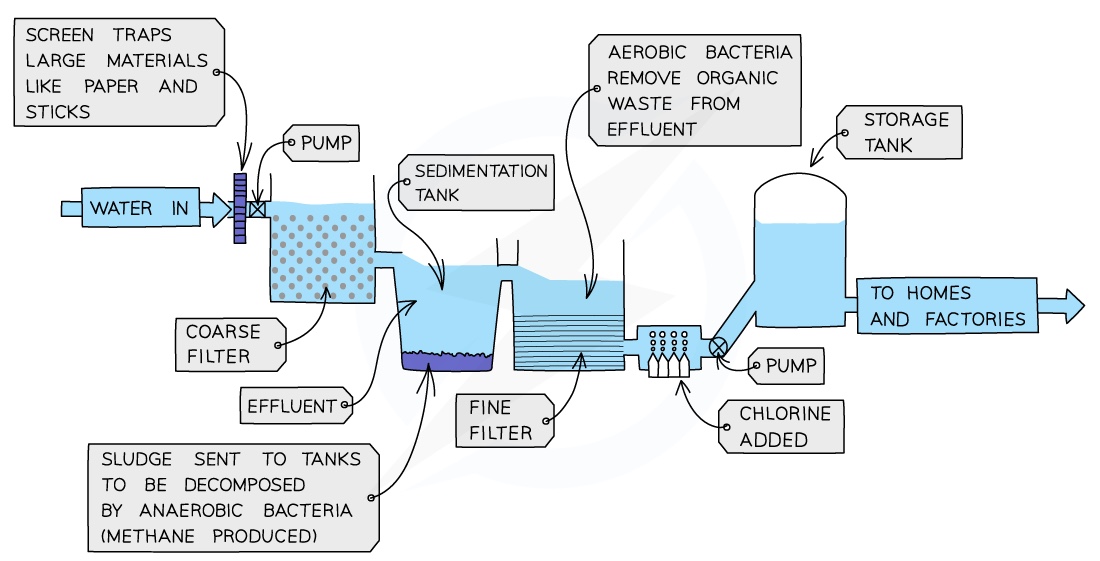
Water treatment
Untreated water contains soluble and insoluble impurities
Insoluble impurities include soil, pieces of plants and other organic matter
Soluble impurities include dissolved calcium, metallic compounds and inorganic pollutants
%%Filtration%% %%is the process used to remove large insoluble particles by passing the water through layers of sand and gravel filters that trap larger particles%%
But ==bacteria and other microorganisms are too small to be trapped by the filters so chlorination is used==
This involves the careful addition of chlorine to the water supply which kills bacteria and other unwanted microorganisms
==Cholera and typhoid are examples of bacterial diseases== which can arise by the consumption of untreated water
Uses of Water
Water in industry
- As a coolant to reduce the temperature of some industrial processes e.g. in nuclear power plants
- Watering crops
- As a solvent in many chemical production processes
- Hydroelectric power stations to generate electricity
- As a first raw material for many processes e.g. the production of ethanol from ethene and steam (water)
Water in homes
- Drinking, cooking and washing
- General sanitation
- In car radiators
- For gardens and plants
An Inadequate supply of water EXTENDED
Clean and safe water supply is very important to mankind
Many problems arise in the event of an inadequate water supply, including:
- Food shortages and famine due to a lack of crops which cannot grow without a clean water supply
- Poor sanitation leads to the spread of bacteria and disease as drinking water becomes infected
13.2 Air
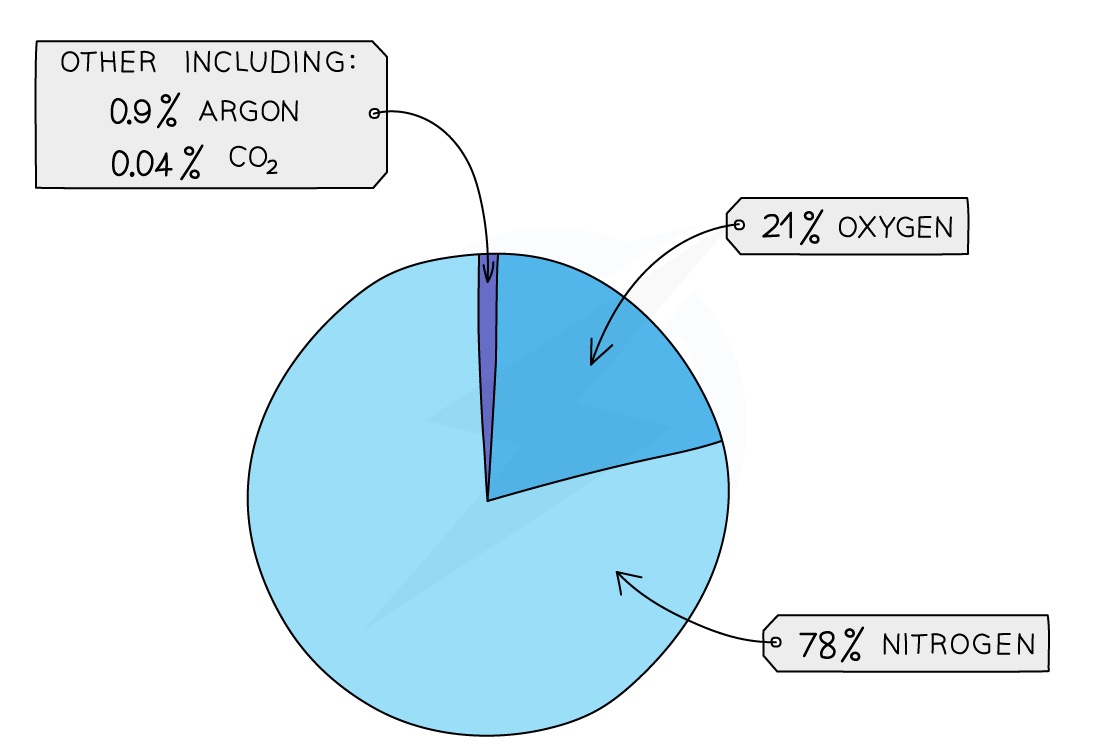
Composition of Air
The chart below shows the approximate percentages by volume of the main gases in unpolluted, dry air:
Uses of air
- The gases available in the air have many important applications
- The noble gases are used in many applications, e.g. helium is used to fill balloons, argon is used in tungsten light bulbs, krypton is used in lasers for eye surgery
- Oxygen is used in steel making, welding and in breathingapparatus
- Nitrogen is used in food packaging, the production of ammonia and in the production of silicon chips
- Oxygen and nitrogen are separated from air by fractional distillation
Investigating the percentage of oxygen in air
- The percentage of oxygen in air can be investigated by passing a known quantity of air over a metal
- The oxygen in the air will react with the metal, forming a metal oxide
- The oxygen will be removed from the air and the volume of the air with the oxygen removed can be measured
- An example of the apparatus that can be used to investigate this is shown below:
Method
Heat the copper using a Bunsen burner
Push the plunger of the syringe containing air, forcing the air into the other plunger until all of the air has transferred
Push the air back from the now filled plunger to the other plunger
Repeat this several times for about 3 minutes
The copper will turn black as copper reacts with the oxygen in the air and copper oxide is produced
Allow the apparatus to cool
Ensure all the gas is in one syringe and record the volume of gas
The percentage of oxygen in air can be calculated from the results

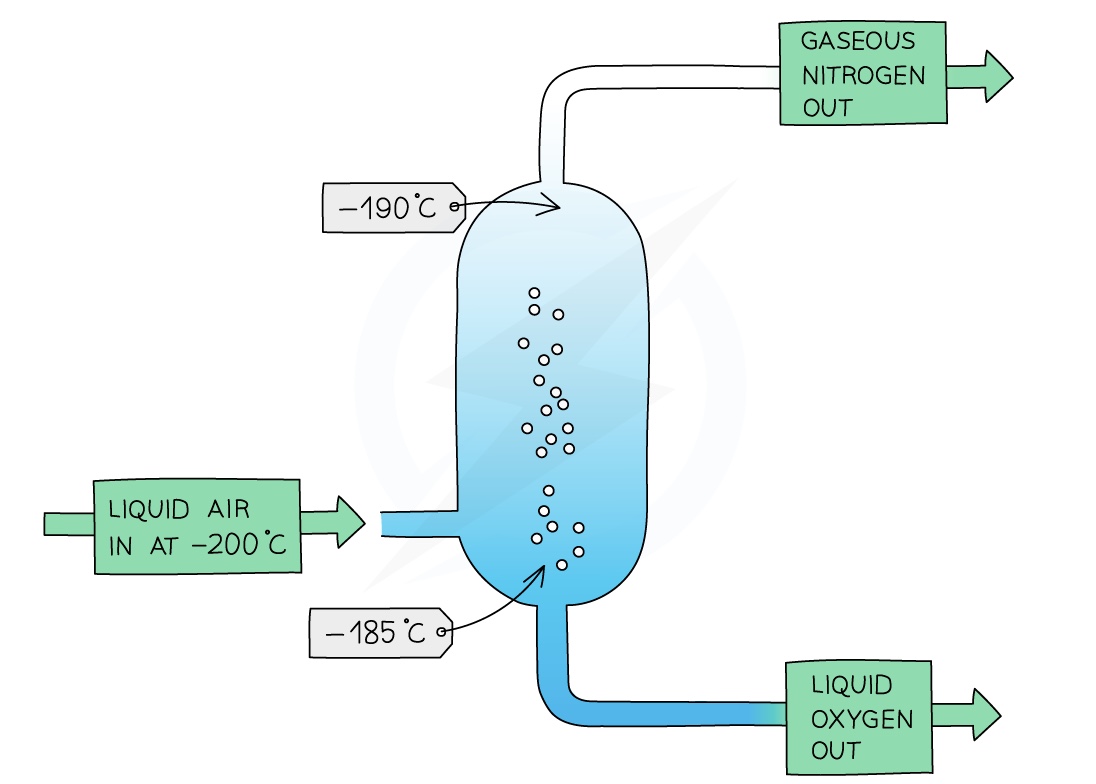
Fractional Distillation of Air EXTENDED
The air is first filtered to remove dust, and then cooled in stages until it reaches –200 °C
Water vapour and carbon dioxide freeze during this cooling process as their melting points are higher than -200 oC and they are removed using filters
The noble gases are still in the gaseous state at -200 ºC, leaving a mixture of liquid nitrogen and oxygen
The liquefied mixture is passed into the bottom of a fractionating column
Note that the column is warmer at the bottom than it is at the top
Oxygen liquefies at -183 °C and nitrogen liquefies at -196 °C
Nitrogen has a lower boiling point than oxygen so it vaporises first and is collected as it rises in the gaseous state to the top of the column
The liquid O2 is then removed from the bottom of the column
11.3 Air Pollution

Carbon Monoxide
- Sources: incomplete combustion of fossil fuels e.g. incomplete combustion of gasoline:
C8H18 + 9O2 → 5CO + 2CO2 + 9H2O
- Adverse effects: poisonous, combining with haemoglobin in blood and prevents it from carrying oxygen
Sulfur dioxide
- Sources: combustion of fossil fuels containing sulfur compounds. Power stations are a major source of sulfur dioxide
- Adverse effects: dissolves in rain to form acid rain which causes corrosion to metal structures, buildings and statues made of carbonate rocks, damage to aquatic organisms. Pollutes crops and water supplies, irritates lungs, throats and eyes and causes respiratory problems
Oxides of nitrogen
- Sources: reaction of nitrogen with oxygen in the presence of high temperatures, e.g. in car engines, high-temperature furnaces and when lightening occurs. It is also a product of bacterial action in soil
- Adverse effects: dissolves in rain to form acid rain with similar effects as SO2 as well as producing photochemical smog
Compounds of lead
- Sources: old water pipes, old paints, petrol in some kinds of racing cars and from very old engines
- Adverse effects: causes significant damage to the central nervous system, young infants are particularly susceptible to lead poisoning causing brain damage
Nitrogen Oxides
- These compounds (NO and NO2) are formed when nitrogen and oxygen react in the high pressure and temperatureconditions of internal combustion engines and blast furnaces
- Exhaust gases also contain unburned hydrocarbons and carbon monoxide
- Cars are fitted with catalytic converters which form a part of their exhaust systems
- Their function is to render these exhaust gases harmless
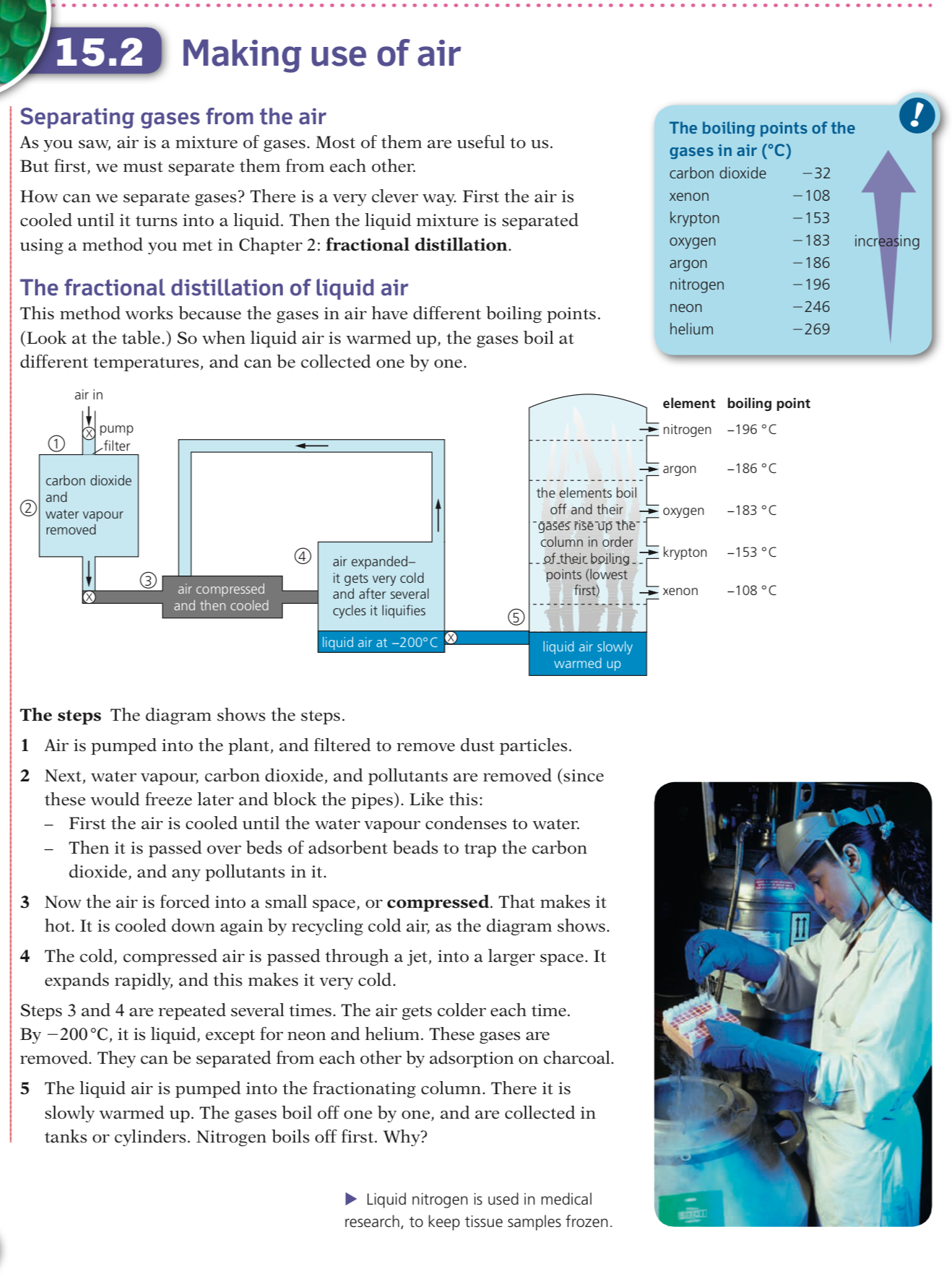
Catalytic converters
They contain a series of transition metal catalystsincluding platinum and rhodium
The metal catalysts are in a honeycomb within the converter to increase the surface area available for reaction
A series of redox reactions occurs which neutralises the pollutant gases
Carbon monoxide is oxidised to carbon dioxide:
- 2CO + O2 → 2CO2
Nitrogen oxides are reduced to N2 gas:
- 2NO → N2 + O2
- 2NO2 → N2 + 2O2
- 4Fe (s) + 3O2 (g) + 4H2O (l) → 2Fe2O3 (reversible sign) 2H2O (s)
A single reaction can summarise the reaction of nitrogen monoxide and carbon monoxide within a catalytic convertor:
- 2NO + 2CO → N2 + 2CO2
Unburned hydrocarbons can also be oxidised to carbon dioxide and water:
- C8H18 + 12½O2 → 8CO2 + 9H2O
11.4 Rusting and Rusting Prevention
The Rusting of iron
- Rust is a chemical reaction between iron, water and oxygen to form the compound hydrated iron(III) oxide (rust)
- Oxygen and water must be present for rust to occur
- Rusting is a redox process where iron is oxidised and it occurs faster in salty water since the presence of sodium chloride increases the electrical conductivity of the water
iron + water + oxygen → hydrated iron(III) oxide
- 4Fe (s) + 3O2 (g) + 4H2O (l) → 2Fe2O3 (reversible sign) 2H2O (s)
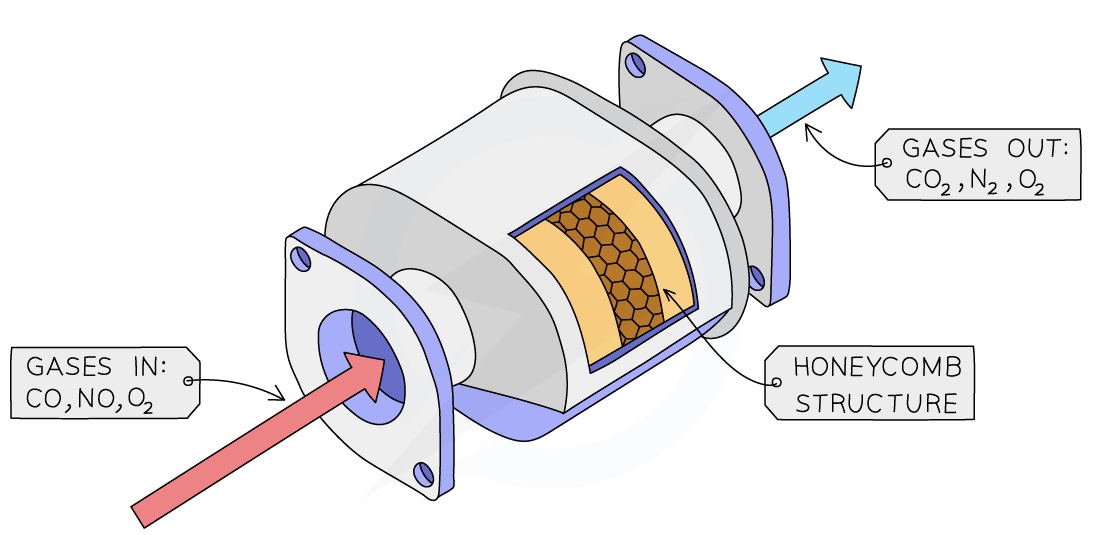
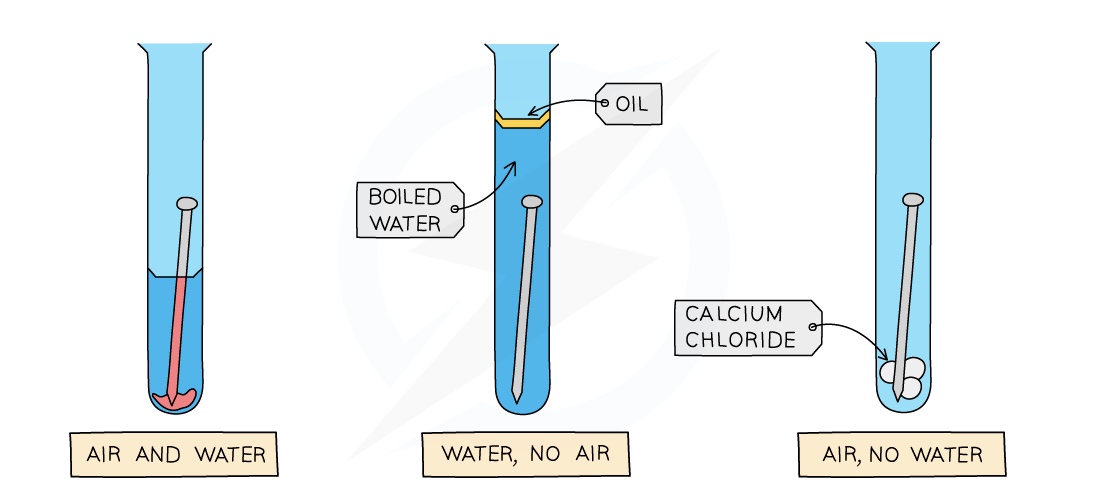
Investigating rusting
To investigate the conditions required for rusting, prepare three test tubes as shown in the diagram
The oil in the 2nd tube keeps out air and the water has been boiled so that no air is left in it
The calcium chloride in the 3rd tube is used to remove any moisture in the air
After a few days, the iron nail in the 1st tube will be the only nail to show signs of rust
Rust Prevention Method
Barrier Methods
Rust can be prevented by coating iron with barriers that prevent the iron from coming into contact with water and oxygen
However, if the coatings are washed away or scratched, the iron is once again exposed to water and oxygen and will rust
Iron can be prevented from rusting using the reactivityseries
^^EXTENDED galvanizing and sacrificial Protection^^
Sacrificial Protection
- A more reactive metal can be attached to less reactive metal
- The more reactive metal will oxidise and therefore corrode first, protecting the less reactive metal from corrosion
- E.g. using magnesium bars on the side of steel ships:
Diagram to show the use of magnesium bars on the sides of steel ships as a method of sacrificial protection
Magnesium is more reactive than iron therefore will oxidise instead of iron:
- Mg → Mg2+ + 2e-
The iron is less reactive therefore will not oxidise and the magnesium is sacrificed to protect the steel
For continued protection, the magnesium bars have to be replaced before they completely corrode
Galvanising
- Galvanising is a process where the iron to be protected is coated with a layer of zinc
- This can be done by electroplating or dipping it into molten zinc
- ZnCO3 is formed when zinc reacts with oxygen and carbon dioxide in the air and protects the iron by the barrier method
- If the coating is damaged or scratched, the iron is still protected from rusting by sacrificial protection