lecture 13: tripartite efflux pumps
tripartite efflux pumps are involved in secretion out of bacteria such as siderophore or defence molecules. In gram negative bacteria, there are two membranes there is a problem of excretion through both membranes so these have evolved for the excretion through the outer membrane as well as the inner. A typical tripartite efflux pump looks like this:
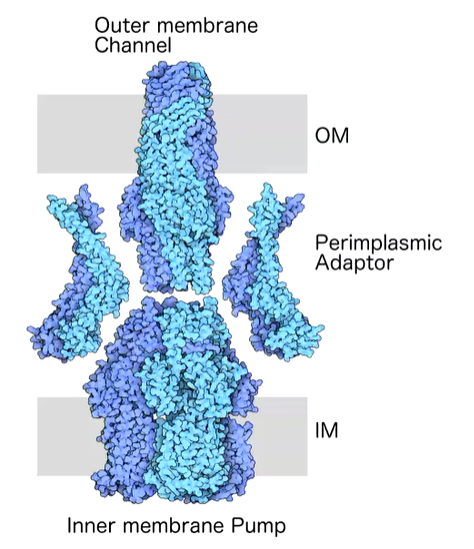
These can have different folds and proteins. There is a long tube like molecule such as TolC which forms a channel through the outer membrane and acts as a long tube out of the membrane. The power of shunting molecules out of the pmp comes from the inner membrane components through dissipation of protein gradients or burning ATP. Both of these power sources are only harnessed from the inner membrane as there is no gradient at the outer membrane. That is the roll of the inner protein. The periplasmic adaptor glues the transporter to the outer membrane protein - TolC.
These pumps can be divided into different families many with TolC. All these families have adaptor proteins with different names as well as tolC but the inner membrane systems are different. The type 1 secretion system is used in e coli to secrete hemolytic toxins. It had the inner membrane component of HLYb WHICH HYDROLYSIS atp to secrete a toxin across both membranes to the outside. This is useful in infections as this toxin can burst cells or other toxic effects. There are other families both are involved in defence against antibiotics.
RND FAMILY PUMP
these pumps move molecules from the periplasmic space to the outside through TolC. MacB pumps picks up molecules from the periplasmic space but can move antibiotics and small proteins and toxins
AcrAB-TolC
This is the archetypal RND family pump and receives substrates from the periplasm and inner membrane but not the cytoplasm. It is powered by proton motive force and has a huge array of substrates such as Dyes, noxious compounds, siderophores, bile salts, detergent resistance and antibiotics
This pump provides resistance to many antibiotics many of which do not have a similar structure but many have ring systems but the overall structures are different. They all however, have the same level of hydrophobicity. It is thought that this pump has evolved to kick out any hydrophobic molecules as these are likely to be antibiotics or things that could kill the bacteria made by other bacteria. The problem is that since there is a variety of substrates AcrAB-TolC can kick out, there is multi drug resistance. Bacteria can acquire a mutation that over expresses the pump so immediately becomes resistance. As it as a wide substrate profile, it will also become resistant to every other antibiotic.
TolC is trimeric and has a beta barrel of 4 antiparallel strands that form a pore in the outer member. There is a channel tunnel along its length. in ISOLATION, tOLc IS CLOSED ON THE periplasmic side:
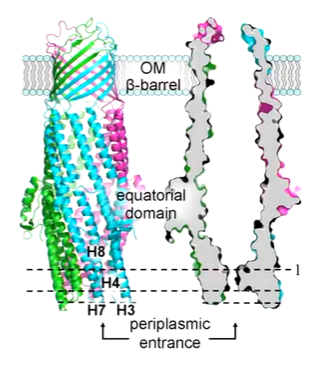
In the absence of the rest of the tripartite machinery, TolC is closed. Hydrogen bonds seal the periplasmic end and TolC thought to be opened by incorporation into the tripartite system. It can be artificially opened by mutating the locking residues that otherwise seal the entrance through hydrogen bonds
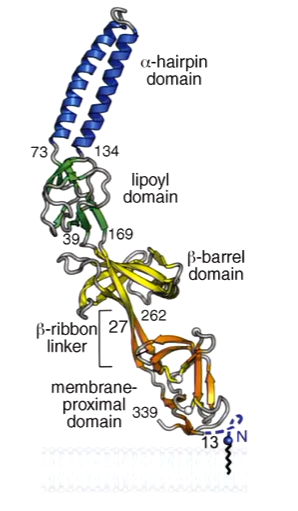
The periplasmic adaptors are 4 linearly arranged domains. They have flexible hinges between doamins. Adaptors are usually tethered to the inner membrane in some way - AcrA has an acyl chain while MacA has a transmembrane helix:
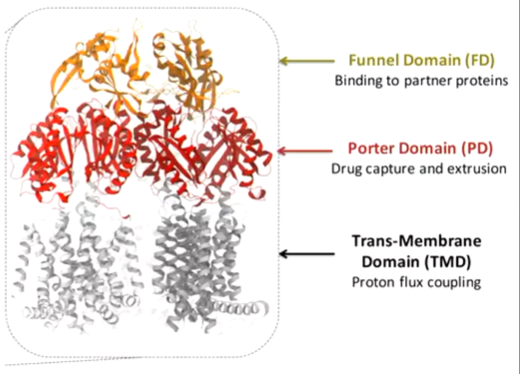
AcrA is the best studied RND family membrane. It is a trimer of three molecules of the AcrB monomer. There are 12 transmembrane helices per monomer - 36 in total. Extensive periplasmic domain is to interact with adaptors. Porter domains are important for binding drugs . There is a funnel domain that is important for binding the adaptors. Proton translocation happens in the transmembrane region.
Cryo-EM used to study AcrAB-TolC and the complex is huge - more than 900 kDa. The structure has an AcrB trimer, AcrA hexamer and a TolC timer - 3:6:3 stoichiometry. Fusion proteins and chemical crosslinking compounds were used to maintain the tripartite assembly. This differs from previous models based on crosslinking that suggest AcrB and TolC contact each other. This complex can be used to find an estimate for how long the periplasmic space is. It was thought the detergent used for stabilization might have disrupted the native structure and so the complexes were prepared in nanodiscs. This meant that there was no crosslinking compounds or artificial protein fusions, This gave a lower resolution but more representative of the pump in vivo. This supported the 3:6:3 stoichiometry.
TolC is closed when substrate is absent and open when it is bound. Minocycline was soaked into the structure and found that although the pump is a trimer, when minocycline is added, it is only bound to one of the monomers. Each of these monomers are in slight different monomers when it is bound.
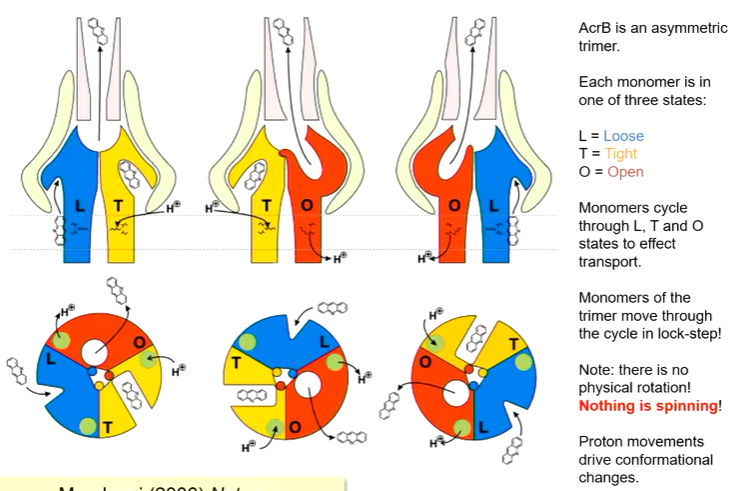
Each monomer is in one of three states - L,T or O. This is a functional rotation mechanism for how the protein works. There is no actual spinning of the monomers and it is not like the ATPase synthase mechanism. The function is moving than the monomers.
The L form is the loose state and has a confirmation that makes it accessible to the periplasm. The T state is bound to a molecule and the O state where the molecule is open at the top where it leads to the adaptor and TolC, It is expected that each time a proton binds, each conformation moves to the next state
It starts with the L state where there is an opening and a molecule can bind. There is movement to the T state due to the movement of proteins. Another protein passes and it moves to the O state where the molecule is open to the adaptor and TolC where it can move through to the TolC. This is also an example of an alternating axis mechanism.