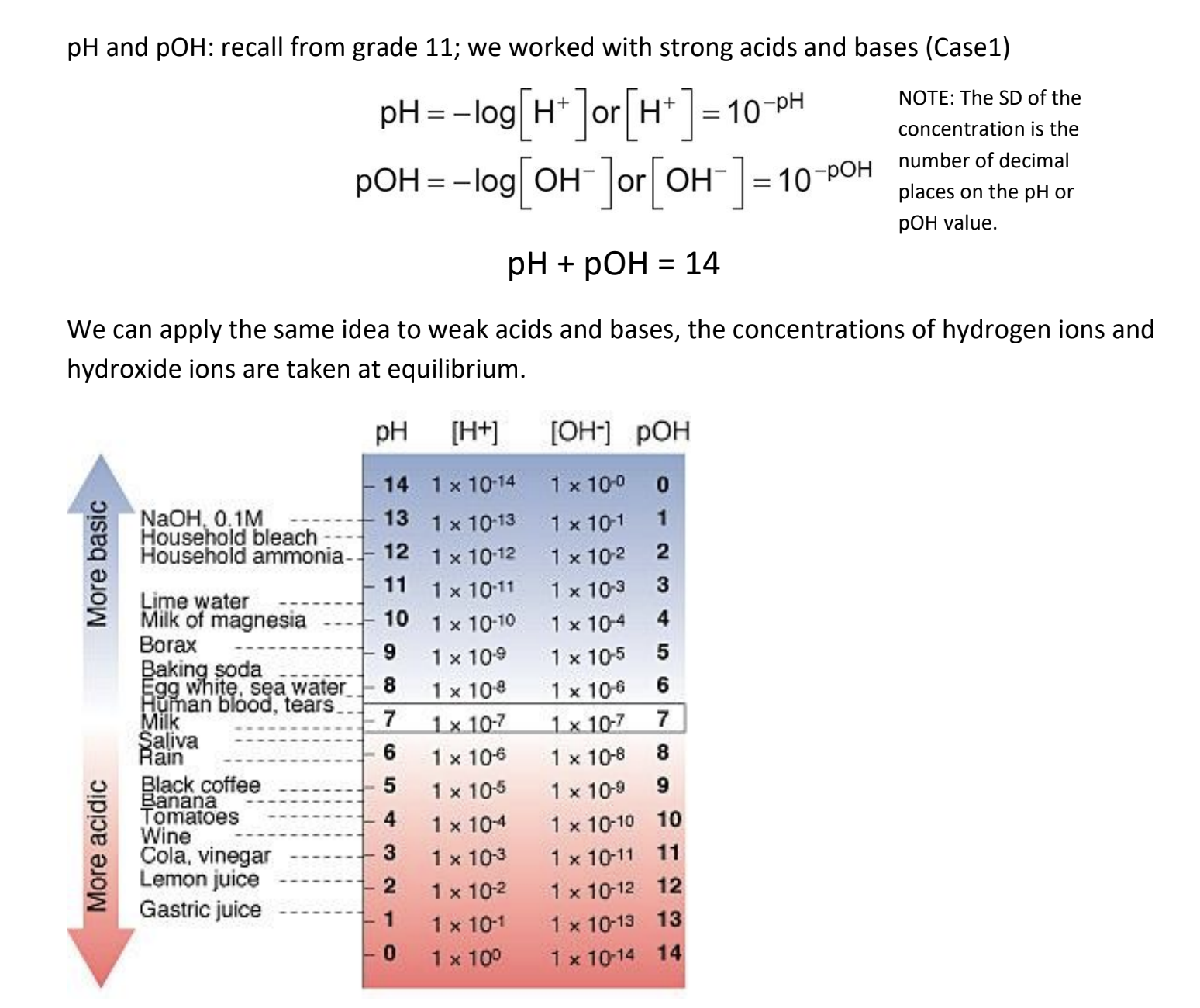
8.2 Strong and Weak Acids and Bases
Acids:
Strong acids/bases: ionize almost to 100% in water, producing hydrogen ions (acids) or hydroxide ions (bases).
Weak acids/bases: only partly ionize in water.
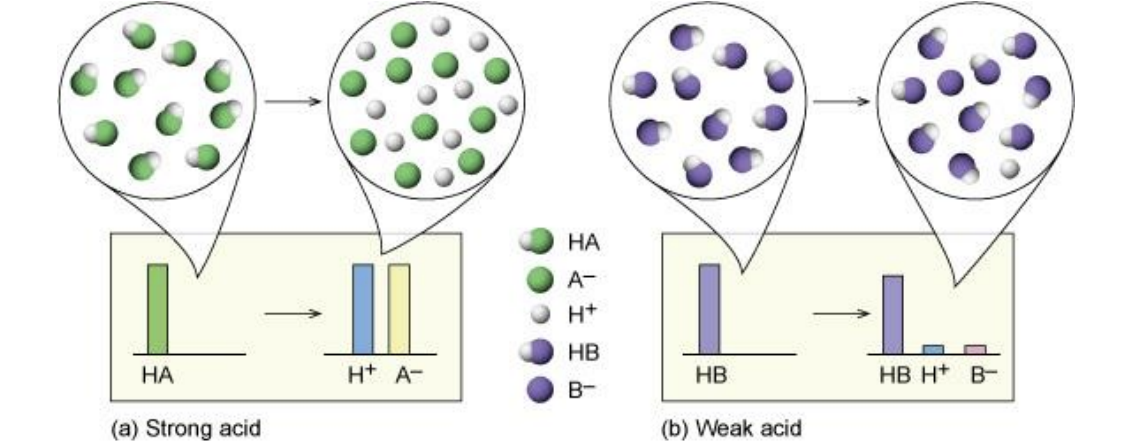
Strong acids have a very large Ka, equilibrium position is far to the right, [HA(aq)]initial [H (aq)]equilibrium
Weak acids have a small Ka, equilibrium position is far to the left, [HA(aq)]initial >> [H+ (aq)]equilibrium
List of Ka values on pg. 496 Table 2 or pg. 726 appendix B5
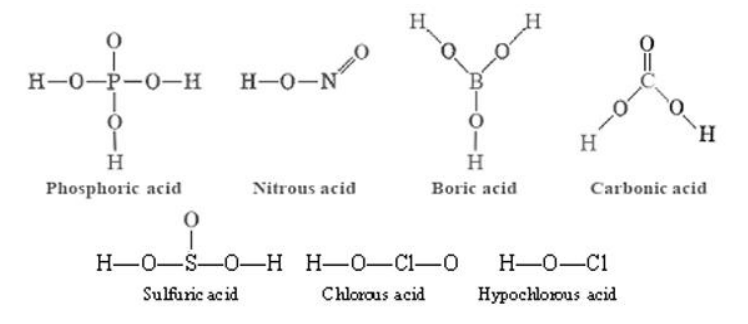
Oxyacids: an acid in which the acidic hydrogen atom is attached to an oxygen atom:
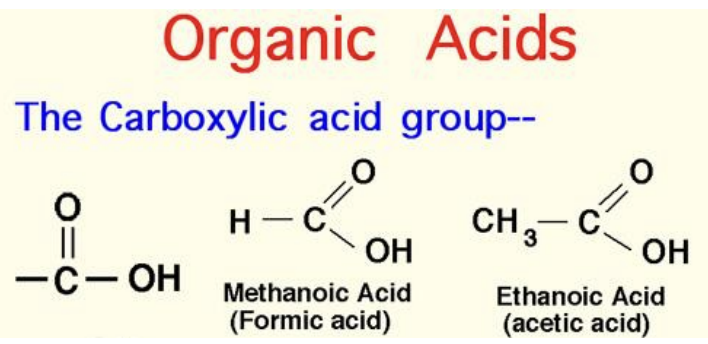
Organic acids: an acids containing a carboxyl group (COOH)
Bases
Strong Bases – hydroxides formed with metals from group I and II on the periodic table. Group
I bases have a high solubility, and high dissociation. We can say that virtually all of the base
dissociates to form the ions:BIG K
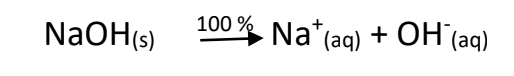
Although group II hydroxides are strong bases, they have low solubility in water:
- Not a lot dissolves, but what dissolves forms a lot of hydroxides. Interesting dichotimies.
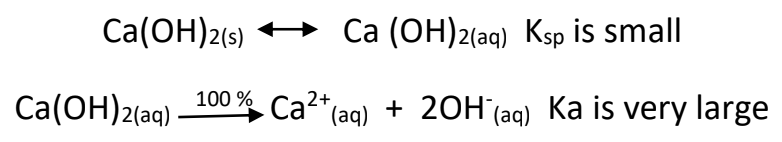
Weak Bases – only partially react to produce hydroxide ions. A good example is ammonia: has
one lone electron pair capable of forming coordinate bonds.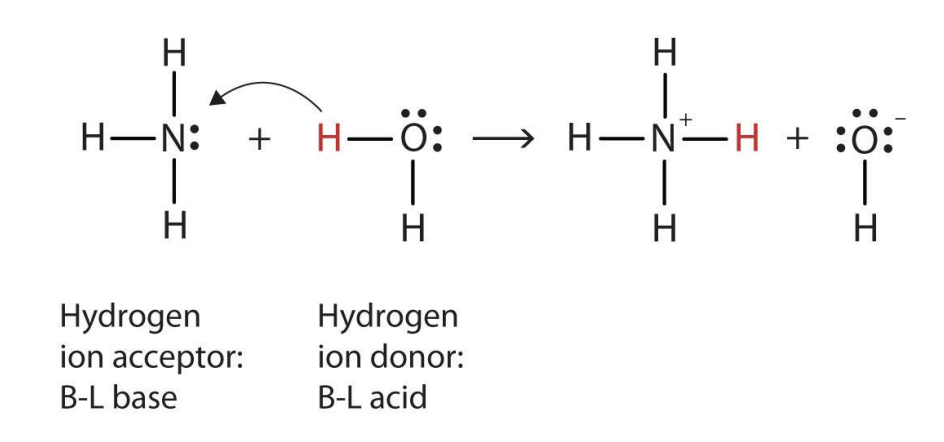
In general, the reaction of a weak base in water is:
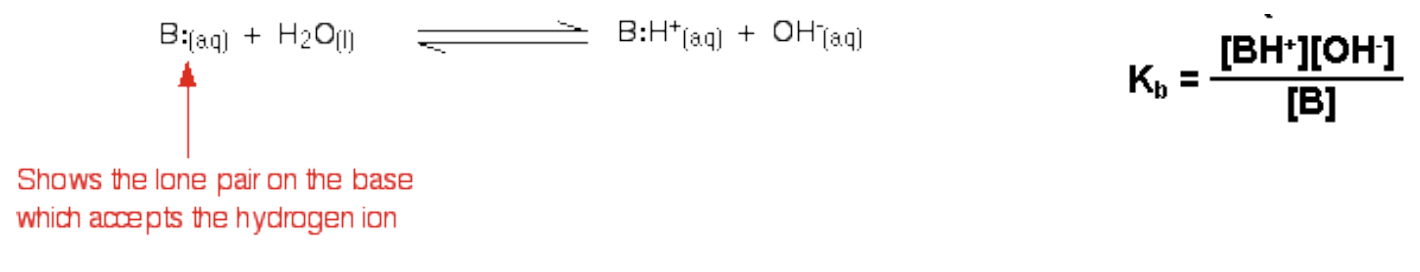
Write the expression for Kb when ammonia reacts with water:

Autoionization of water: the transfer of a hydrogen ion from one water molecule to another:
- This is called the ion-product constant for water (Kw)
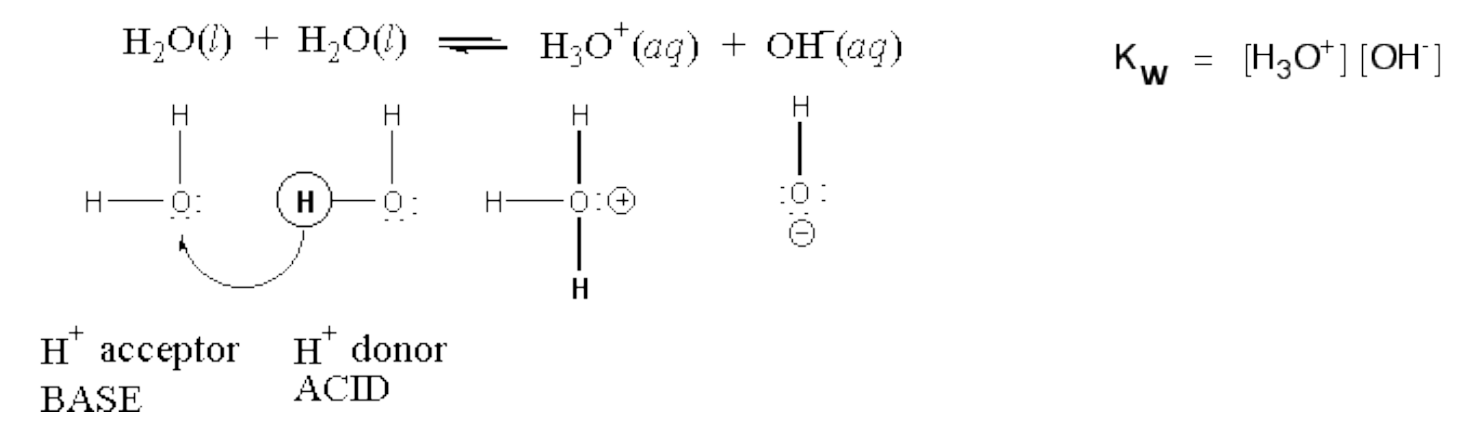
At 25 oC, [H+ (aq)] is 1.0 x 10-7 mol/L and [OH- (aq)] is 1.0 x 10-7 mol/L. Calculate Kw:
- Kw = )concentration of hydronium)(concentration of hydroxide)
- 1.0 x 10^-14
- Always the equilibrium constant for the autoionization of water
Three situations:
- Kw = KaKb

Overall, as the strength of the acid increases, strength of the conjugate base decreases, and
vice versa:- A strong acid/base will have a very weak conjugate (only forward rx)
- A weak acid/base will have a weak conjugate (equilibrium)
- A very weak acid/base will have a strong conjugate (no reaction)
If you need concentrations: