Chemical Bonding notes
4.1 - Ionic Compounds
Ion: An Atom with a charge.
2 types of Ions: Cations (positive), and Anion (Negative).
Elements become Ions to have a complete outer shell (to become stable).
Octet rule with a few exceptions. Ie hydrogen (H)
IONIC BONDING
In an Ionic compound, atoms GAIN or LOSE electrons to be stable.
Happens between a Metal (Cation) and a Nonmetal (Anion).
One atom is more attracted to electrons than others and will steal/take its valence electrons.
Electronegativity: the attraction between nucleus and valence electrons.
Structural Formula
Covalent
Ionic picture
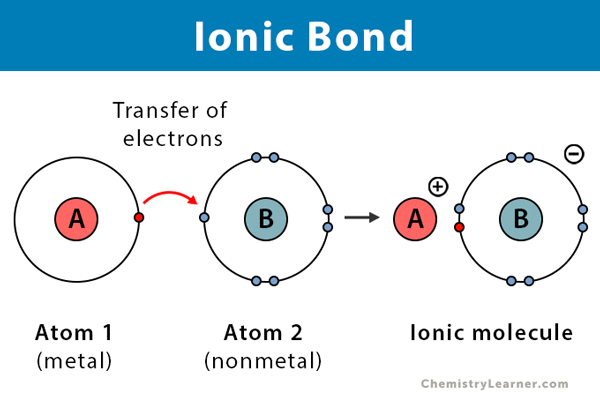
IONIC BONDING PROPERTIES
Forms a crystal lattice structure
High melting/boiling points
Hard and rigid
Do NOT conduct electricity as solids
4.2 - Metallic Bonding
Happens between 2 Metals
Interaction between delocalized electrons and the metal nuclei.
Delocalized: when valence electrons are freely moving ( not local to any one metal atom)
Forms a lattice of positive ions in a “sea of electrons” -> Metal atoms donate their valence electrons to form a “sea”
Electrons are freely moving
Malleable and ductile
4.3 - Covalent Bonding
Happens between 2 nonmetals (anions)
Both nonmetal atoms will have high electron affinity (Electronegativity) which will cause the atoms to SHARE their electrons
IONIC BONDS
Metal + Nonmetal
Electrons transfer
Higher melting/boiling points
STRONGEST BOND
COVALENT BONDS
Nonmetal + Nonmetal
Electrons shared
Low melting/boiling point
Types Of Covalent Bonds
Single Covalent Bonds H - H
Double Covalent Bonds O = O
Triple Covalent Bonds N ≡ N
A triple bond is stronger, cuz it has more electrons being shared
Each bond represents 2 electrons being shared.
DIATOMIC ELEMENTS
Diatomic elements are elements that exist in nature as 2 covalently bonded atoms
Like cl - cl br - br fl - fl o = o
Hydrogen - H^2
Nitrogen - N^2
Oxygen - O^2
Fluorine - F^2
Chlorine - Cl^2
Bromine - Br^2
Iodine - I^2
VSEPR MODEL: Valence Shell Electron Pair Repulsion
Shows the 3-D structure of covalent Bonds
Linear - 2 atoms on the central atom
Bent - 2 atoms on central atom + 2 lone pairs
Trigonal Planar - 3 atoms on central atom
Trigonal Pyramidal - 3 atoms on central atom + 1 lone pair
Tetrahedral - 4 atoms on the central atom
Lone Pair - pair of electrons not used in bond
You don't gotta know the angle I think
Polarity
In polar covalent bond, electrons aren't shared equally
One element is pulling harder due to electronegativity
Nonpolar covalent bonds share electrons equally
Polar covalent bonds do NOT share electrons equally
Find the difference between polarity
EN diff = 0 ------> nonpolar covalent bond
EN diff = 0.1-1.7 --------> polar covalent bond
EN diff = > 1.7 (more than 1.7) -----> Ionic bond
Polar molecules have both partial positive and partial negative poles
This depends on
- Structural formula/ shape of molecule
- Polar bonds
- Separation of charge into oppositely charged poles
You can also use the flow chart (green = yes, red = no)
4.5 - Naming Binary Ionic Bonds
Cation First, then Anion
Add -Ide on the Anion
NaCl
Sodium Chlorine → Sodium Chloride
Writing Binary Ionic Formulas
The scientific term for the charge is oxidation number
Crisscross to write the bonds in a formula
When writing binary ionic compounds
Net charge = 0
Positive ion first
Cross charges to get subscript hi miwin :3
POLYATOMIC IONS
When naming: Cation + Polyatomic Ion
Name both as is on the chart (NO IDE)
Put parentheses when using more than one PA Ion
Treat PA as one unit, do NOT change subscripts inside the parentheses
MULTIVALENT IONIC BONDS
Aka funny metals, need Roman numerals to indicate the charge
Name the funny metal (cation) as it appears
Find charge for funny metal using REVERSE CRISS CROSS (use the subscripts to find charge)
Use Roman numerals (ie II, III, IV) after the funny metal to show its charge
Name anion using ide, if PA then write PA as is
NAMING COVALENT COMPOUNDS
Needs a prefix (ie carbon dioxide, CO2)
1 - Mono
2 - Di
3 - Tri
4 - Tetra
5 - Penta
6 - Hexa
7- Hepta
8 - Octa
9 - Nona
10 - Deca
The first element will have a prefix unless it is only one, use a normal name, (no ide on first element)
NEVER use mono on the first atom
The second atom ALWAYS has a prefix
EXAMPLES
S4O2 - Tetrasulfur Dioxide
P205 - Diphosphorus PentoxidePentaoxide
NH3 - Nitrogen Trihydride
NAMING ACIDS
Starts with H (hydrogen) because it dissolves in water
If binary (H and a nonmetal)
Use the prefix hydro and change the end of nonmetal to -ic
Ie HBR - Hydrobromic Acid
H2S - Hydrosulfuric Acid
IF POLYATOMIC IS INVOLVED
Don't use the prefix hydro
If PA ends in ATE change to IC “I ATE something gross and said IC”
Ie HNO3 (hydrogen + nitrate) - Nitric Acid
If PA ends in ITE change to OUS “venomOUS snakes bITE”
Ie HNO2 ( hydrogen + nitrite) - Nitrous acid