lecture 2 - DESIGN OF SKELETAL MUSCLE
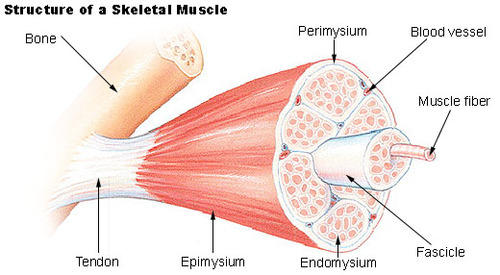
whole muscle surrounded by epimysium (connective tissue)
fascicles (group of fibers) surrounded by perimysium
myofiber surrounded by endomysium (overlays sarcolemma - muscle fiber cell membrane)
like saran wrap**
**contractile apparatus consists of parallel cross-striated myofibrils
SLIDING FILAMENT THEORY **myofibrils responsible for contraction
Myosin
larger (500kd), thick filament
features globular heads acting as ATPase (determines rate of contraction)
M line in middle of myosin molecule, heads in each side move toward M line (bipolar)
its structure allows it to form filaments with motor heads oriented in opposite directions at each end, enabling it to generate contractile force by pulling on actin filaments simultaneously in opposing directions
Actin
smaller (42kd) called thin filament
2 intertwined subunits (forms grooves → has troponin and tropomyosin)
no ATPase
terminates at Z-line (sarcomere)
Z to Z line = 1 sarcomere
H zone gets smaller when contracting
** no inherent directionality when contracting; both sides of sarcomere pull in to middle
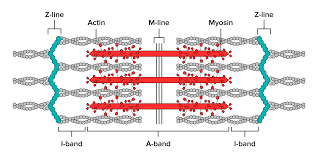
** myosin heads cover whole myosin not just top and bottom
wrapped around actin is Tropomyosin
associated with tropomyosin is troponin
troponin consists of 3 subunits
TnI - bound to actin - inhibits interaction between actin anf myosin
TnT - bound to tropomyosin - anchors troponin complex to actin
TnC - bound to calcium - calcium sensor that triggers conformational change
tropomyosin physically blocks the myosin binding sites on actin when calcium levels are low; when calcium levens increase it binds to TnC and triggers a conformational change that moves tropomyosin away, allowing myosin to bind to actin and powerstroke to initiate muscle contraction
**ATP doesn’t do the contraction - it just releases the potential energy PE
***********************short answer??
4 FEATURES OF MUSCLES
exciteability - ability of a muscle to respond to a stimulus
extensibility - muscle's capacity to be stretched or extended without tearing.
elasticity - muscle's ability to return to its original length after being stretched.
contractility -The muscle's ability to shorten and produce force when stimulated.
each myosin surrounded by 6 actin
each actin surrounded by 3 myosin
in space → muscles get weaker bc there’s no gravity
loss of strength not just from atrophy → lose alot more strength than mass
what happens is that stroichiometry changes
measures 1:5 or 1:2
reduces the # of actin-myosin cross-bridges
**tendon connects muscle to bone
fasciculi/fasciculitis - (different fiber types in the fasiculi)
pull out 1 fasicle
pull out a single fiber - striation from myosin and actin
pull out 1 myofibril - myofibrils don’t get bigger they just increase in number (fibers get bigger)
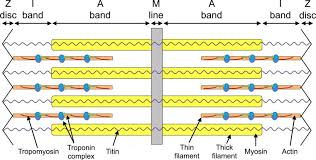
sarcomere - smallest functional unit of muscle
contractile protein: myosin, actin
regulatory protein: troponin, tropomyosin
titin
plays a role in how much force you can produce
on the end of myosin and binds myosin to Z line - strong anchor fiber
produces 20-25% of the overall force during??
during contraction:
I band reduced, A band has no change, H zone reduced
******************************************THIS IS ON SHORT ANSWER
SLIDING FILAMENT THEORY OF MUSCLE CONTRACTION
nervous system → action potential propagates to SR; calcium released from SR binds to TnC
Causes conformational shift that is transmitted to rest of troponin and to tropomyosin
tropomyosin buried deeper in groove between 2 strands of actin, then exposing binding (active) sites
energized myosin cross bridge head (ADP & Pi bound) binds to exposed actin binding site
releases stored energy in crossbridge head and powerstroke occurs pulling actin over myosin
during power stroke ADP and Pi released from cross bridge head
increase or decrease in affinity depends on the release of Pi from crossbridge head (inc. in affinity when Pi is released)
At the end of power stroke, crossbridge head stays attached to actin until a new ATP molecule binds to crossbridge head of myosin breaking acto-myosin complex
ATPase of crossbridge head immediately hydrolyzes ATP; leaving crossbridge head in energized position ( snapped back to original position) with ADP and Pi head.
Proccess continues (cross bridge cycling) until cytosolic calcium returns to normal and actin binding sites are masked again
In any fiber, cross bridge cycling occurs asynchronously (allows tension to be maintained rather than cycles off and on)
meaning different myosin heads attach and detach from actin filaments at slightly different times, which allows for continuous tension generation and smooth muscle contraction rather than a jerky on-and-off cycle.
same speed but not the same time for smooth contraction
ATP provides possibility of power stroke - not power stroke directly
**shortening has no direction
**power stroke happens before release of ADP and Pi
the two are not released together
when Pi is still attached → weak binding
vs strong binding

Rigor Mortis
can’t get rid of Pi → because there is no new ATP molecule
the myosin head stays cocked
ATP is depleted, the cross-links between actin and myosin become irreversible. This results in the muscles remaining permanently contracted,
Role of Ca2+ in contraction

AP propagates down T-tubule
the DHP receptor senses a voltage change and signals the ryanodine receptor to release calcium ions from the SR
SR calcium pump is always on - actively pumping calcium ions into SR
but it becomes overwhelmed when an AP causes massive release of ca from SR → significant increase in calcium concentration