AP Chem Unit 2
2.1 Types of Chemical Bonds
electronegativity values for the representative elements increase going from left to right across a period and decrease going down a group valence electrons shared between atoms of similar electronegativity constitute a nonpolar covalent bond
valence electrons shared between atoms of unequal electronegativity constitute a polar covalent bond
atoms with the higher electronegativity will develop a partial negative charge relative to the other atom in the bond
in single bonds, greater differences in electronegativity lead to greater bond dipoles
all polar bonds have some ionic character
difference between ionic and covalent bonding is not distinct, but rather continuum
difference in electronegativity is not only factor in determining if a bond should be considered ionic or covalent
bond between metal an nonmetal = ionic
bond between two nonmetals = covalent
examination of the properties of a compound is best way to determine type of bonding
in metallic solid, valence electrons from metal atoms are considered to be delocalized and not associated with any individual atom
ionic bonds formed between two ions by transfer of electrons
polar covalent bonds are unequal sharing of electrons between atoms in a molecule
nonpolar covalent bonds are equal sharing of electrons between atoms in a molecule
pure covalent bond has < 0.4 electronegativty difference
polar covalent bond has between 0.4-1.8 electronegativity difference
ionic bond has > 1.8 electronegativity difference
2.3 Structure of Ionic Solids
cations and anions in an ionic crystal are arranged in a systematic, period 3D array that maximizes the attractive forces among cations and anions while minimizing the repulsive forces
Increasing Electronegativity Difference Between Bonding Atoms
Nonpolar covalent
little to no difference
2 of the same atoms
C and H
Polar covalent
moderate electronegativity difference
2 different nonmetals
Ionic
large electronegativity difference
1 metal, 1 nonmetal
Ionic Bonds
between atoms of metals and nonmetals with very different electronegativity
cation is positive and anion is negative
bond formed by transfer of electrons
form crystalline solids
produce charged ions all states
conductors
high melting point
Crystalline solids (salts)
hard, but brittle
specific pattern means if it is disrupted they will shift and shatter
lot of ionic bonds in slat
takes more energy to break them apart
Lattice Energy
lattice energy is the energy required/released to form the crystal lattice structure of ionic compounds
the greater the attractive forces, the more negative the lattice structure energy
negative means energy released when it is formed or the energy required to break it
big charge = big attraction = high potential energy
small ions = big attraction = high potential energy
2.4 Structure of Metals and Alloys
metallic bonds form between metals and metals
metallic bonds happen between metals with similar electronegativity
sea of electrons is shared between nucleus
compounds are not made
alloys are mixtures of metals
substitutionally and interstitial alloys are based on the size of the metals atoms
Properties of Metals
reflective
high conductivity of electricity and heat
malleable (ability to be beaten into a flat sheet)
ductile
Metallic Bonding
electron cloud around atoms
electrons are delocalized
formed between atoms of metallic elements
good conductors at all states
lustrous
very high melting point
Electron Flow
when an electrical current is applied to the metal electrons can flow easily from one mental atoms to another in the direction of the current
leads to man metal sample's’ ability to conduct electricity
Metals Form Alloys
metals do not combine with metals, they form alloys which is a solution of a metal in a metal
alloys are homogeneous mixture of metals made by combining two or more metallic elements
goal of making an alloy is usually to give greater strength, resistance to corrosion or other desirable properties
2.5 Lewis Diagrams
Covalent Bond Notes
between nonmetals with similar electronegativity
bond formed by sharing electrons
several electrostatic interactions
attraction between electrons and nucleus
repulsions between electrons
repulsions between nuclei
Predicting Structure of Covalent Bonds
lewis structure for single atoms
shows only the valence electrons
place one electron on each of four sides of atom betfore making pairs
Octet Rule
atoms prefer to have eight valence electrons
reason that atoms bond and form molecules is to create octet
exception: H only required a duet ( 2 electrons )
lewis structures account for all the electrons and attempt to meet the octet rule
General Reminders
Hydrogen only has one orbital
single, double and triple bonds exist
triple are the shortest
there are exceptions to octet rule
Lewis Dot Structures
add up the valence electrons
polyatomic ions assign an extra electron for each negative charge, subtract electron for each positive charge
select central atom and connect all atoms with single bond
central atom = Carbon or least electronegative atom
complete the octet of all atoms
unbonded electrons should be in pairs
check electrons
if you run out of electrons use multiple bonds
if there are extra electrons, put them on central
expanded octet
if molecule has net charge, place the structure in brackets with the charge in the upper right corner
Notable Rules
sometimes the ordered list may help
HCl
Central atoms is often written first
H’s and F’s are terminal (on outside always)
C’s love to hook together and do not like to be terminal
avoid unshared pairs on carbons
oxygens do not bond together
except in O2 and peroxide molecules
avoid rings of three or fewer atoms
Expanded Octets
elements with three or more energy levels have unused orbitals which allow for expanded octets
must be on n = 3 of p table or lower
only applies on the central atom of a molecule
maximum number of domains is 6
Polyatomic Ions
have charge, which means the amount of electrons are effected
when you complete a Lewis structure of polyatomic ion, place the structure in brackets and place the charge on the upper right corner
Isomers
molecules with the same chemical formula, but different arrangements of atoms
2.6 Formal Charge and Resonance
Formal Charge
hypothetical charge the atom would have if we could redistribute electrons in bonds evenly between the atoms
subtracting nonbonding electrons, then subtract number of bonds connected to that atom in Lewis structure
formal charge = number of valence shell electrons (free atoms) * number of lone pair electrons - ½ number of bonding electrons
sum of formal charge of all atoms in molecule must be zero
in an ion should equal charge of ion
formal charge ≠ actual charge
used as bookkeeping procedure
no indication of actual charge
helpful with determining isomers
lewis structure with formal charge closest to zero
Resonance
triple bond > double bond > single bond
if ≥ 2 lewis structures with same atom arrangement can be written for molecule/ion, actual distribution of electron is average shown from various lewis structures
individual lewis structures "resonance forms”
actual electronic structure of molecule called resonance hybrid of individual resonance forms
doe not fluctuate between resonance forms
always the average shown
dotted line indicates that some models have bond there, but not all
solid line indicates all models have bond there
Formal Charge Summary
all atoms must be counted
sum of all formal charges in a neutral molecule must be zero
sum of all formal charges in a n ion must equal the charge of the ion
smaller the formal charge on an individual, the better
when formal charge is needed, negative formal charges should go on the most electronegative atom
positive formal charges should go on the least electronegative atom
molecular structure in which all formal charges are zero is preferred
if must have nonzero formal charges, the arrangement with the smallest non-zero formal charge in preferred
lewis structures are preferable wen adjacent formal charges are zero or the opposite sign
when choosing from several lewis structures with similar distributions of formal charge, the structure with the negative charges on the more electronegative atoms is preferred
2.7 VSPER and Hybridization
VSPER
valence shell electron pair repulsion theory
steps
count electron domains
single/double/triple bonds count as 1
predict shape
arrangement of electron groups around central atom (electron geometry), exclude lone pairs (molecular geometry)
VSPER chart
must memorize
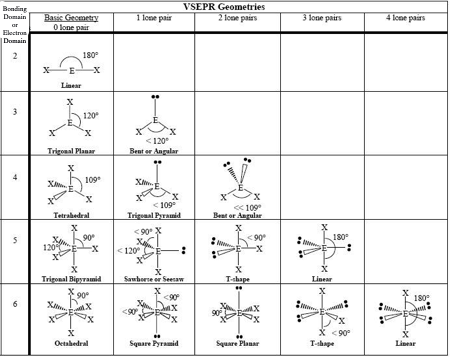
Hybridization and Dipole
number of electron domains | electron geometry | hybridization of bonded orbitals |
2 | linear | sp |
3 | trigonal planar | sp2 |
4 | tetrahedral | sp3 |
5 | trigonal bipyramidal | sp3d |
6 | octahedral | sp3d2 |