Chapter 1: Review
1.1 Intro
Organic compounds are derived from living organisms like plants and animals
inorganic compounds are derived from nonliving sources like minerals and gases
Organic compounds have different properties;
difficult to isolate and purify
decompose easier in heat than inorganic
vitalism (19th century) proposed that this was due to a ‘vital force’ that organic compounds contained that inorganic compounds didn’t (disproven in 1828 when a German chemist converted ammonium cyanate (inorganic salt) to urea (an organic component of urine).
Organic compounds are now defined as compounds containing carbon atoms
inorganic compounds are those lacking carbon atoms
1.2 The Structural Theory of Matter
Suggests that substances are defined by a specific arrangement of atoms
For example (C2H6O) in the form of dimethyl ether (H3COCH3) and ethanol (H3CCH2OH)
though they have the same molecular formula, they differ in connectivity and are called constitutional isomers
constitutional isomers have different physical properties and names.
each element will generally form a predictable number of bonds
tetravalent; forming four bonds (e.g. carbon)
trivalent; forming three bonds (e.g. nitrogen)
divalent; forming two bonds (e.g. oxygen)
monovalent; forming one bond (e.g. hydrogen)
1.3 Electrons, Bonds, and Lewis Structures
Bonds
Bonds connect atoms together
covalent bonds are the result of two atoms sharing a pair of electrons
A bond between two H+ atoms has a change in H=-436 KJ/mol, meaning that there is a net decrease in energy through the bond’s formation
There are several forces contributing to energy in bond formation; repulsion between neg electrons, repulsion between pos nuclei, and attraction between pos and neg charges. Electrons move to minimize repulsion and maximize attraction.
The lowest energy state is the most stable, and determines bond length and strength
Lewis Structures
Represent shared valence electrons and lone pairs in molecules and individual atoms
Lewis Dot structures indicate the number of valence electrons for a given atom.
octet rule; atoms form bonds to achieve a full valence shell of either 2 (hydrogen) or 8 electrons in order to emulate the noble gases.
1.4 Formal Charges
associated with atoms that don’t exhibit the appropriate number of valence electrons and must be indicated in a lewis structure.
can be determined from the correct number of valence electrons vs the actual number.
bonds count as 1 electron and lone pairs count as 2
ex. Carbon with four bonds has 4 valence electrons. The correct number is 4, 4-4=0, and therefore the carbon atom doesn’t have a formal charge.
1.5 induction and polar covalent bonds
the three categories of bond are covalent, polar covalent, and ionic. The type of bond is calculated from the difference in electronegativity values of the participating atoms
electronegativity is the measure of an atoms ability to attract electrons
Roughly speaking, if the difference is less then 0.5, then the bond is covalent. If the difference is between 0.5 and 1.7, then the bond is polar. And if the difference is greater than 1.7, the bond is ionic.
Covalent bonds equally share electrons while the more electronegative atom of a polar covalent bond holds most of the electron density. This is called induction
Ionic bonds don’t share electrons at all and are instead formed from the attraction of a positively and negatively charged atom. Therefore, strictly ionic bonds can’t be drawn as a line but just the two atoms near each other with their respective charges indicated.
Ionic vs polar covalent or polar vs covalent aren’t strict rules because of the variability in electronegativity values
electrostatic potential maps show electron density distribution across molecules with red zones indicating negative and blue zones indicating positive charges.
1.6 Bond-Line Structures
bond line structures are more convenient ways to draw organic molecules then lewis structures which show the molecules as a series of zig-zag lines with heteroatoms indicated by letter.
corners or endpoints indicate a carbon
single lines indicate a single bond, double lines a double bond, and triple lines a triple bond.
Carbon atoms are assumed to have as many hydrogen atoms needed to fulfil their octet unless otherwise indicated by heteroatoms, double/triple bonds, and formal charges.
1.7 Atomic Orbitals
quantum mechanics
electrons exhibit wavelike behavior
the wave equation describes the total energy of a hydrogen atom while taking the wavelike behavior into account.
the wave equation is solved into a series of solutions called wavefunctions which are denoted by a psi symbol and the appropriate number.
This shows how electrons exist at discrete energy levels (energy is quantized)
wavefunctions are a function of spatial location that allows us to assign a value for each location in space relative to the nucleus.
psi square indicates the probability of finding an electron in a location
Electron density and atomic orbitals
An orbital is the space an electron can occupy, which can be thought of as a cloud of electron density
types of orbitals are s, p, d, and f
phases of atomic orbitals
waves have negative/positive psi values (position around the node) and the node is where psi=0
a negative/positive psi value indicates position around the node, not charge for atomic orbitals
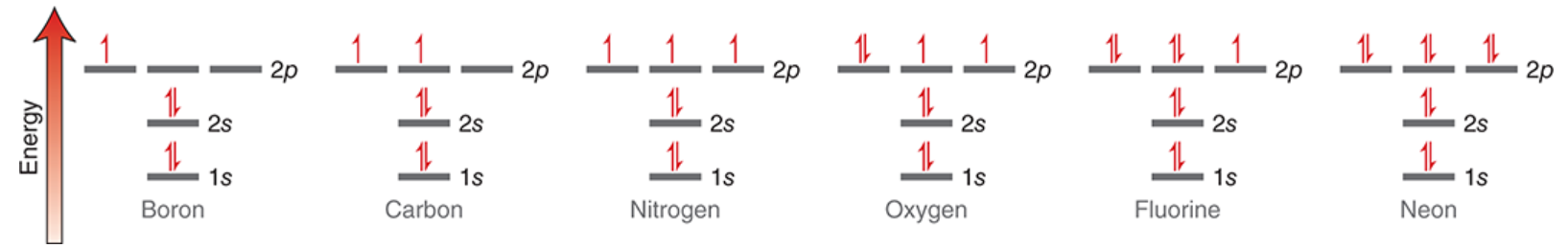
filling atomic orbitals with electrons
electrons have lower (starting with 1s) or higher energy level depending on what orbital they occupy.
degenerate orbitals have the same energy levels (i.e. each of the 3 2p orbitals are degenerate)
aufbau principle says that the lowest energy orbital is filled first
the pauli exclusion principle says each orbital can have 2 electrons with opposite ‘spin’
hund’s rule says that when dealing with degenerate orbitals, each orbital receives one electron before electrons are paired up
energy diagrams illustrate these principles
1.8 Valence bond theory
covalent bonds are formed from the overlap of atomic orbitals
Valence bond theory and molecular orbital theory explain this concept
There are two possible outcomes when waves interact, constructive (waves reinforce each other and increase amplitude) or destructive (waves cancel each other out and produce a node) interference
valence bond theory says that a bond is shared electron density as a result of constructive interference from two atoms atomic orbitals.
i.e. the bond between two hydrogen atoms is the result of their 1s orbitals overlapping. The electron density is focused primarily on the bond axis (a line drawn between the two atoms) and the bond is called a sigma bond
all single bonds are sigma bonds. Sigma bonds are characterized by circular symmetry around the bond axis
1.10 Hybridized Atomic Orbitals
sp³ hybridization (methane example)
electron configuration can’t describe the bonding structure of methane (carbon with four separate C-H bonds) because this configuration only shows two atomic orbitals capable of that type of bond
furthermore, the geometry of a free carbon atom isn’t the same as the tetrahedral CH_4.
we can mathematically average (hybridize) the 2s and three 2p orbitals to give four degenerate hybridized orbitals. (mathematical procedure not physical process)
these orbitals are called sp³ hybridized orbitals which are indicated on an energy diagram as four spaces at an energy level higher than 2s but slightly lower than 2p. The hybridized orbitals have an unsymmetrical p shape
double bonds and sp² hybridization
in the formation of a double bond (for example, ethylene H_2C=CH_2), each carbon atom only forms three bonds and only needs three hybridized orbitals.
So the s orbital is only hybridized with two of the p orbitals and the remaining p is unaffected
this leaves three orbitals available for sigma bonding. The remaining p orbitals can interact for a separate interaction called a pi bond
the sigma and pi bond between the carbon atoms is what causes the double bond
triple bonds and sp hybridization
similar to the sp², sp hybridization is the result of one s and one p orbital, leaving two p orbitals available for pi bonding
bond strength and length
the increasing amount of bond interactions with each hybridization level results in an increasing bond strength with each number of bonds.
also decreasing bond length
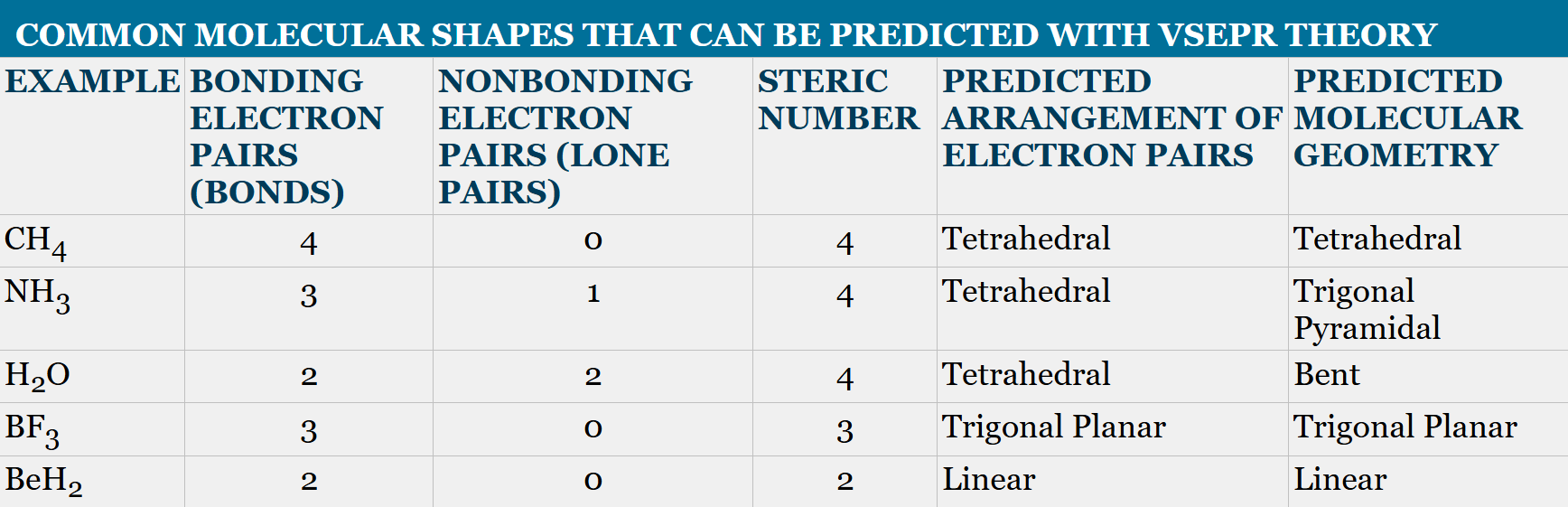
1.11 predicting molecular geometry with VSEPR theory
using the nature of electrons to repel each other, we can make quick predictions about molecular geometry.
called valence shell electron pair repulsion (VSEPR theory)
Tetrahedral geometry
exact bond angles of 109.5°
sp³ hybridization
trigonal pyramidal geometry
the total number of electron pairs is called the steric number which is 4 for this case and the previous
however instead of four sigma bonds, there are three and one lone pair so the molecule arranges for the electron pair to be as far as possible from the others, resulting in a pyramid shape
bond angles are approximately 107° because lone pairs repel more strongly than sigma bonds
bent geometry
H_2O has two sigma bonds and 2 lone pairs which equals a steric number of 4
instead of a tetrahedral arrangement, however, the lone pairs push the bond angle to 105°
trigonal planar geometry
BF_3 is sp² hybridized, meaning it has three sigma bonds and an empty p orbital
all bond angles are 120°
the steric number is 3
linear geometry
BeH_2 is sp hybridized meaning it has only 2 sigma bonds
the bond angle is 180°
1.12 Dipole moments and molecular polarity
a molecule is said to exhibit a dipole moment when an inductive effect is caused by the presence of an electronegative atom in the molecule
dipole moment is an indicator of polarity and is calculated from the amount of partial charge on either side of the dipole multiplied by distance of separation
dipole moments are stated in terms of debye (D)
calculating dipole moments allows us to state how ionic a bond is
for molecules with multiple polar bonds, you have to take the vector sum of the dipoles which is called the molecular dipole moment
2dipoles in opposite directions cancel out while dipoles in the same direction augment each other.
1.13 intermolecular forces and physical properties
intermolecular forces are the attractive forces between molecules and they determine the physical properties of a compound
all intermolecular forces are electrostatic — the attractive forces between opposite charges
the three kinds electrostatic interactions between neutral molecules are;
dipole-dipole
hydrogen bonding
fleeting dipole-dipole
dipole-dipole interactions
in the solid state; aligned so opposites attract to each other more than same repels
for liquid and gas; decreasing changes of alignment respectively
increased dipole moment strength elevates melting and boiling point
hydrogen bonding
not actually a bond but an attractive force
from a hydrogen atom that is attached to an electronegative atom (O,N, or F) and has a partial positive charge through induction that is attracted the lone pair of an electronegative atom.
a very strong interaction due to the relatively small size of hydrogen that allows the partial charges to get very close. The strength drastically changes the boiling point of a compound
extremely important in biological compounds in determining shapes and interactions.
fleeting dipole-dipole interactions
since electrons are always moving, so is electron density and partial charge
so at any given moment, a temporary dipole moment can form that forms an attractive force to neighboring molecules
also called London dispersion forces.
strength of these forces depends on molecule surface area.
generally, higher molecular weight has a higher boiling point
1.14 solubility
like dissolves like; polar compounds are soluble in polar solvents and vice versa.
this is because of the presence or lack of dipole-dipole interactions between the solvent and solute.
soap
soaps have one polar end and one nonpolar end called the hydrophilic and hydrophobic region respectively.
nonpolar molecules (like oil) are surrounded by the nonpolar ends with the polar ends facing outwards to form a micelle which renders the nonpolar substance water (a polar solvent) soluble
dry cleaning
it is simpler sometimes just to use a nonpolar solvent then forming a micelle
this allows clothes to be cleaned without coming into contact with water or soap.