
2.4.1 Enzymes
An enzyme is a biological catalyst, typically a protein, that speeds up chemical reactions in living organisms by lowering the activation energy needed for the reaction to proceed, without being consumed in the process. Enzymes are highly specific to their substrates due to their unique active sites and can be regulated by various factors, including temperature, pH, and the presence of inhibitors or activators.
Enzymes:
Biological catalysts made up of globular proteins.
Catalyse metabolic reactions by reducing Ea without being used up.
Unique and highly specific active sites; only form ESC’s w/ complementary substrates, due to their tertiary structures.
Catalyse reactions of 2 types:
Extracellular: - Catalyse reactions outside of cells. (eg. Trypsin; hydrolyses proteins in small intestine. Optimum pH: 7.5-8.5 or Salivary Amylase; hydrolyses starch into maltose.)
Intracellular: Catalyse reactions within cells. (eg. Catalase; works within liver cells breaking down H2O2 into O2 and H2O. Optimum pH: in humans 7 however varies between pH 4-11 in other species.)
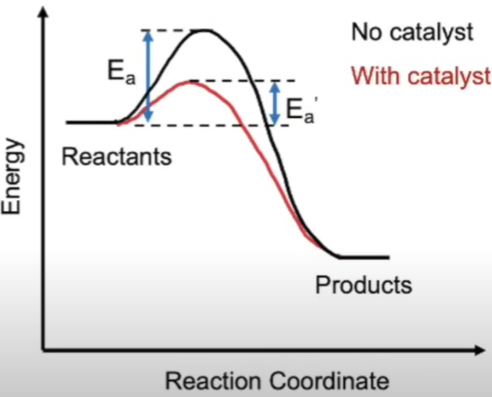
Activation Energy (Ea):
When enzymes bind to their specific complementary substrates they form Enzyme-substrate-complexes (ESC’s).
In doing this, they lower the Ea required for reaction to occur thus increasing no. of molecules with sufficient energy.
2.4.1 Enzymes
An enzyme is a biological catalyst, typically a protein, that speeds up chemical reactions in living organisms by lowering the activation energy needed for the reaction to proceed, without being consumed in the process. Enzymes are highly specific to their substrates due to their unique active sites and can be regulated by various factors, including temperature, pH, and the presence of inhibitors or activators.
Enzymes:
Biological catalysts made up of globular proteins.
Catalyse metabolic reactions by reducing Ea without being used up.
Unique and highly specific active sites; only form ESC’s w/ complementary substrates, due to their tertiary structures.
Catalyse reactions of 2 types:
Extracellular: - Catalyse reactions outside of cells. (eg. Trypsin; hydrolyses proteins in small intestine. Optimum pH: 7.5-8.5 or Salivary Amylase; hydrolyses starch into maltose.)
Intracellular: Catalyse reactions within cells. (eg. Catalase; works within liver cells breaking down H2O2 into O2 and H2O. Optimum pH: in humans 7 however varies between pH 4-11 in other species.)
Activation Energy (Ea):
When enzymes bind to their specific complementary substrates they form Enzyme-substrate-complexes (ESC’s).
In doing this, they lower the Ea required for reaction to occur thus increasing no. of molecules with sufficient energy.

 Knowt
Knowt