Acid & Bases
The magnitude of Ka indicates the tendency of an acid to ionize in water. Since [H+] is found in the numerator of the equilibrium constant expression for the dissociation of an acid, a stronger acid will have a larger Ka val
Weak acids
Citric acid (C8H6O7) is a weak acid
Acetic acid is a weak acid
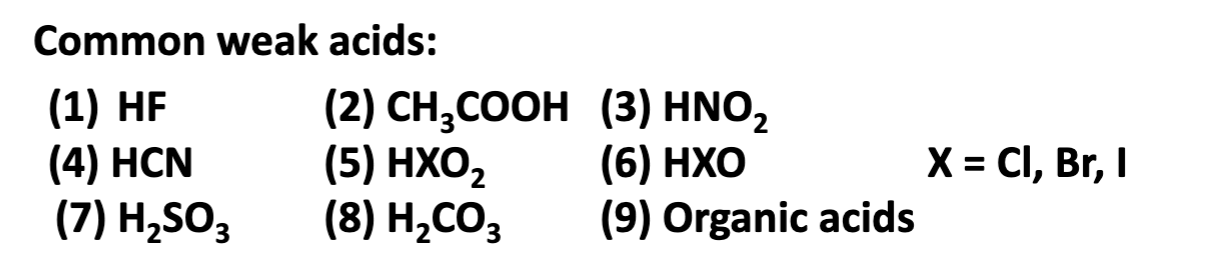
Common weak acids:
HF
HCN
H2SO3
CH3COOH (acetic acid)
HXO2
H2CO3
HNO2
HXO
Organic acids —> carbon carbon
X = Cl, Br, I
K = (Ka*Kb)/Kw
Big sized atom bonded to H leads to weaker and longer chemical bond, which results in a higher acidity due to the increased ability of the acid to donate protons.
In contrast, smaller atoms like fluorine form stronger bonds with hydrogen, resulting in lower acidity as they hold onto protons more tightly.
increasing ionic radius gives more volume to spread -1 charge over & minimize electron-electron repulsions
what does the ICE box stand for? Initial, Change, Equilibrium concentrations
% dissociation = [conjugate base equilibrium]/ [acid] initial * 100
pH decreases as [H3O+) increases
amphoteric = can act as both base & acid
water is amphoteric
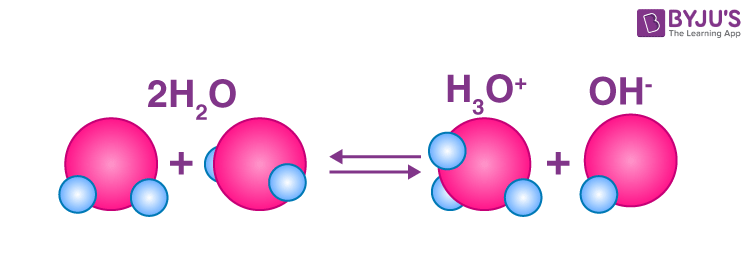
Solution is neutral
[H3O+]=[OH-]
Acidic
[H3O+] > [OH-]
Basic
[H3O+] < [OH-]
Why does water auto-ionization occur? because of entropy
Water autoionizlewation is endergonic. Reactants are favored
Lewis acid = accepts pair of electrons
Lewis base = donates pair of electrons
Bronsted dowry acid = donates proton
Bronsted dowry base = accepts proton