
Organic Chemistry Introduction IGCSE Chemistry
Introduction
Organic Chemistry is the scientific study of the structure, properties, and reactions of organic compounds. Organic compounds are those which contain carbon
For conventional reasons metal carbonates, carbon dioxide and carbon monoxide are not included in organic compounds
Definition of a Hydrocarbon
A compound that contains only hydrogen and carbon atoms
An alkane is an example of a hydrocarbon. In this section, we will mention alkanes, however we will go into more detail on them in a following section.
Representing Organic Molecules
Organic compounds can be represented in a number of ways:
Empirical Formulae
Molecular Formulae
General Formulae
Structural Formulae
Condensed Structural Formulae
The empirical formula shows the simplest possible ratio of the atoms in a molecule
For example: Hydrogen peroxide is H2O2 but the empirical formula is HO
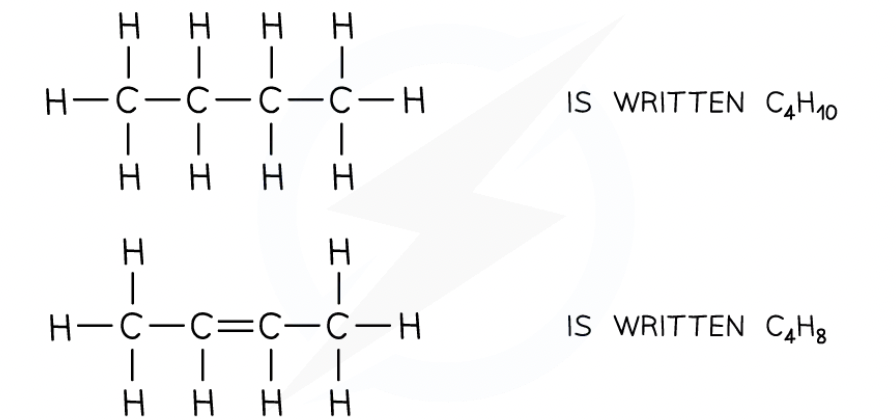
The molecular formula shows the actual number of atoms in a molecule
For example:
The general formula shows a ratio of atoms in a family of compounds in terms of ‘n’ where n is a varying whole number
For example, the general formula of a molecule that belongs to the alkane family is CnH2n+2
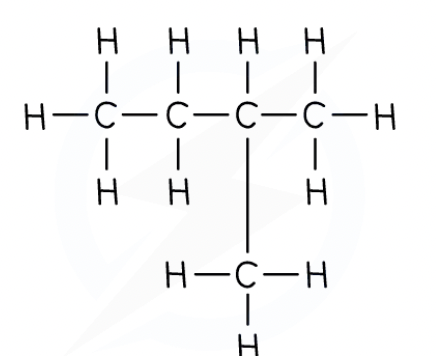
The displayed formula shows the spatial arrangement of all the atoms and bonds in a molecule
This is also known as the graphical formula.
For example:
In a structural formula, enough information is shown to make the structure clear, but most of the actual covalent bonds are omitted
Only important bonds are always shown, such as double and triple bonds
Identical groups can be bracketed together
Side groups are also shown using brackets
Straight chain alkanes are shown as follows:

Organic Terminology
Three important terms to know in this topic are homologous series, functional group and isomerism
Homologous Series
This is a series or family of organic compounds that have similar features and chemical properties due to them having the same functional group
All members of a homologous series have:
The same general formula
Same functional group
Similar chemical properties
Gradation in their physical properties
The difference in the molecular formula between one member and the next is CH2
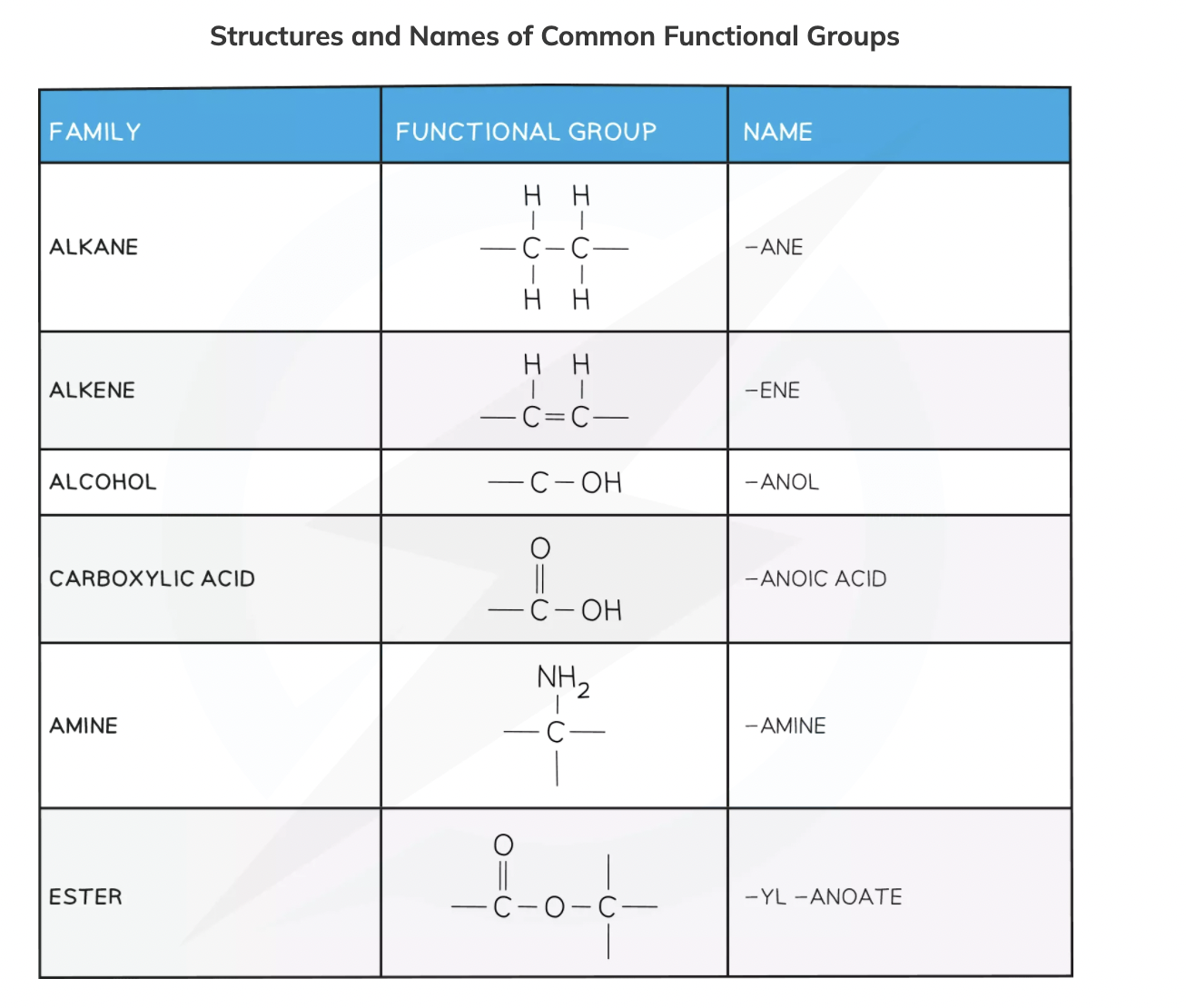
Functional Group
Functional group: A group of atoms bonded in a specific arrangement that influences the properties of the homologous series
Some examples are shown here
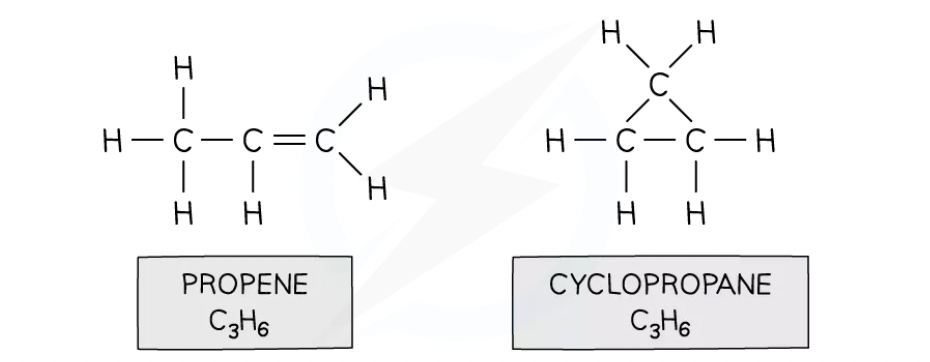
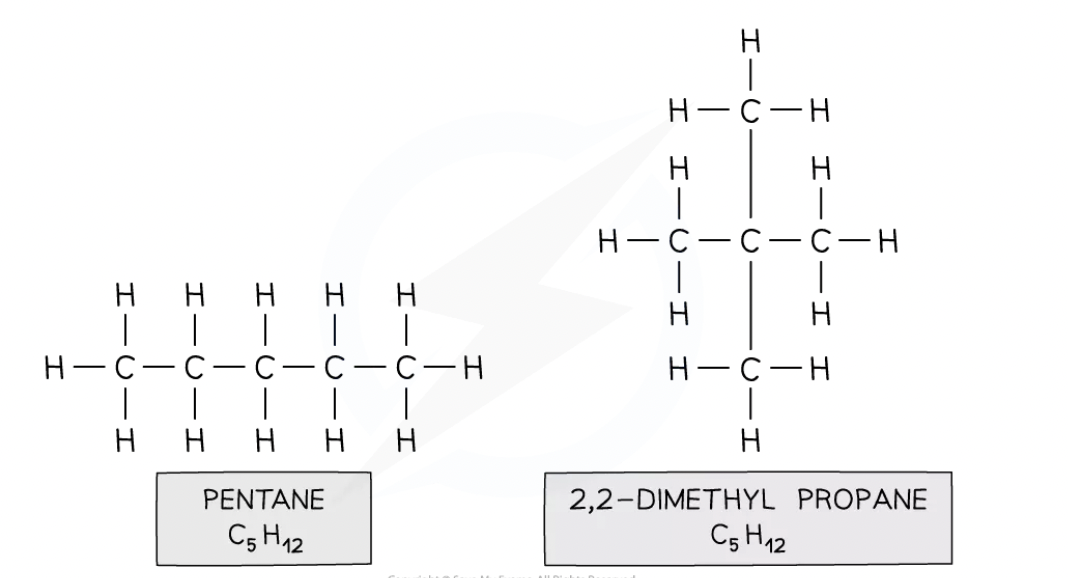
Isomerism
Isomers are compounds that have the same molecular formula but different displayed formulae
Eg. propene and cyclopropane
Names of compounds
The names of organic compounds have two parts: the prefix or stem and the end part (or suffix)
The prefix tells you how many carbon atoms are present in the longest continuous chain in the compound
The suffix tells you what functional group is on the compound

Further rules for naming compounds
When there is more than one carbon atom where a functional group can be located it is important to distinguish exactly which carbon the functional group is on
Each carbon is numbered and these numbers are used to describe where the functional group is
When 2 functional groups are present di- is used as a prefix to the second part of the name
Classifying Organic Reactions
The reactions of organic compounds can be classified into substitution, addition and combustion
Substitution
A substitution reaction takes place when one functional group is replaced by another
Example: Methane reacts with bromine under ultraviolet light
CH4 + Br2 → CH3Br + HBr
Methane + Bromine → Bromomethane + Hydrogen Bromide
Addition
An addition reaction takes place when two or more molecules combine to form a larger molecule with no other products
Example: Bromine will react with ethene and the bromine molecule will react and add across the double bond of the ethene
C2H4 + Br2 → C2H4Br2
Ethene + Bromine → Dibromoethane
Combustion
This is the scientific term for burning. In a combustion reaction, an organic substance reacts with oxygen to form carbon dioxide (or carbon monoxide if incomplete combustion) and water.
Example: Alkenes burn when heated in air of oxygen
If there is an unlimited supply of air / oxygen, the products are carbon dioxide and water:
CH4 + 2O2 → CO2 + 2H2O
C3H8 + 5O2 → 3CO2 + 4H2O
This is termed complete combustion
If there is a limited supply of air / oxygen, the products are carbon monoxide and water:
CH4 + ½O2 → CO + 2H2O
This is termed incomplete combustion
Organic Chemistry Introduction IGCSE Chemistry
Introduction
Organic Chemistry is the scientific study of the structure, properties, and reactions of organic compounds. Organic compounds are those which contain carbon
For conventional reasons metal carbonates, carbon dioxide and carbon monoxide are not included in organic compounds
Definition of a Hydrocarbon
A compound that contains only hydrogen and carbon atoms
An alkane is an example of a hydrocarbon. In this section, we will mention alkanes, however we will go into more detail on them in a following section.
Representing Organic Molecules
Organic compounds can be represented in a number of ways:
Empirical Formulae
Molecular Formulae
General Formulae
Structural Formulae
Condensed Structural Formulae
The empirical formula shows the simplest possible ratio of the atoms in a molecule
For example: Hydrogen peroxide is H2O2 but the empirical formula is HO
The molecular formula shows the actual number of atoms in a molecule
For example:

The general formula shows a ratio of atoms in a family of compounds in terms of ‘n’ where n is a varying whole number
For example, the general formula of a molecule that belongs to the alkane family is CnH2n+2
The displayed formula shows the spatial arrangement of all the atoms and bonds in a molecule
This is also known as the graphical formula.
For example:

In a structural formula, enough information is shown to make the structure clear, but most of the actual covalent bonds are omitted
Only important bonds are always shown, such as double and triple bonds
Identical groups can be bracketed together
Side groups are also shown using brackets
Straight chain alkanes are shown as follows:

Organic Terminology
Three important terms to know in this topic are homologous series, functional group and isomerism
Homologous Series
This is a series or family of organic compounds that have similar features and chemical properties due to them having the same functional group
All members of a homologous series have:
The same general formula
Same functional group
Similar chemical properties
Gradation in their physical properties
The difference in the molecular formula between one member and the next is CH2
Functional Group
Functional group: A group of atoms bonded in a specific arrangement that influences the properties of the homologous series
Some examples are shown here

Isomerism
Isomers are compounds that have the same molecular formula but different displayed formulae
Eg. propene and cyclopropane

Names of compounds
The names of organic compounds have two parts: the prefix or stem and the end part (or suffix)
The prefix tells you how many carbon atoms are present in the longest continuous chain in the compound
The suffix tells you what functional group is on the compound

Further rules for naming compounds
When there is more than one carbon atom where a functional group can be located it is important to distinguish exactly which carbon the functional group is on
Each carbon is numbered and these numbers are used to describe where the functional group is
When 2 functional groups are present di- is used as a prefix to the second part of the name

Classifying Organic Reactions
The reactions of organic compounds can be classified into substitution, addition and combustion
Substitution
A substitution reaction takes place when one functional group is replaced by another
Example: Methane reacts with bromine under ultraviolet light
CH4 + Br2 → CH3Br + HBr
Methane + Bromine → Bromomethane + Hydrogen Bromide
Addition
An addition reaction takes place when two or more molecules combine to form a larger molecule with no other products
Example: Bromine will react with ethene and the bromine molecule will react and add across the double bond of the ethene
C2H4 + Br2 → C2H4Br2
Ethene + Bromine → Dibromoethane
Combustion
This is the scientific term for burning. In a combustion reaction, an organic substance reacts with oxygen to form carbon dioxide (or carbon monoxide if incomplete combustion) and water.
Example: Alkenes burn when heated in air of oxygen
If there is an unlimited supply of air / oxygen, the products are carbon dioxide and water:
CH4 + 2O2 → CO2 + 2H2O
C3H8 + 5O2 → 3CO2 + 4H2O
This is termed complete combustion
If there is a limited supply of air / oxygen, the products are carbon monoxide and water:
CH4 + ½O2 → CO + 2H2O
This is termed incomplete combustion
 Knowt
Knowt