All chapter notes
Chapter 1
Biology- the scientific study of life
Cells- the structural and functional units of life
There are seven characteristics of life:
Order- life is categorized by highly ordered structures
Response to the environment- All organisms respond to stimuli from their environment
Regulation- Organisms have mechanisms to maintain homeostasis
Growth and development- The DNA organisms inherit controls their pattern of growth. Organisms get bigger and change
Energy processing- Organisms take in energy and use it to power their activities
Evolutionary adaptation- Traits evolve over generations to help live their environments better
Reproduction- Organisms reproduce with their own kind
Homo sapiens- Wise man
Taxonomy- Branch of bio that names and classifies species
Hierarchy of life
kingdom
phylum
class
order
family
genus
There are five kingdoms:
bacteria
protist
plants
fungi
animal
There are three domains
Bacteria
Archaea
Eukarya includes:
protists
plants
fungi
animals
Hierarchic of organizations of life
Organelle
Celle
Tissue
Organ
Organ system
Organism
Population
Community
Ecosystem
Biosphere
Science- an approach to understanding the natural world
Data- the evidence that is used to base a scientific question
Hypothesis- a proposed explanation for a set of scientific questions
Experiment- a scientific test
Theory- A broader scope of hypothesis supported by a large body of evidence
Controlled experiment- an experiment where one of the experimental group is compared to a control group.
Independent variable- the factor manipulated by the researcher
Dependent variable- affected by the independent variable
Variable- any condition that may cause change in the system that is being studied
Feedback mechanisms- used by organisms to maintain or amplify chemical systems
Two types:
Positive feedback
increases stimulus
Negative feedback
Decreases stimulus
maintains homeostasis
Stimulus- triggers a reaction
Signal- communication usually between 2 systems
Response- how something reacts to a stimulus
Evolution- the idea that living species are descendants of ancestral species that were different from the present day ones
Natural selection- A process in which certain inherited traits are more likely to survive and reproduce than the individuals that don’t have those traits
Humans affect evolution intentionally or unintentionally
Artificial selection- the selective breeding of domesticated plants and animals to promote the occurrence of specific traits
The processes of life depend on the transmission and use of information.
Genes- A discrete unit of hereditary Information consisting of a specific nucleotide sequence in DNA
Gene expression- The process where genetic information flows from genes to make a protein
In biology structure (the shape of something) and function (what it does) are related and are used to give information about each other
The activities of life require energy
Vital parts of the ecosystem are small animals, fungi and bacteria in the soil that decomposes water.
The input and conversion of energy form one form to another make life possible
Energy flows though a system in one direction: entering as light and exiting as heat
Life depends on the interactions within different systems
System- the complex organization of the components of life.
Systems biology- An approach to studying biology that aims to model the dynamic behavior of biological systems based on a study of their interactions among their parts
Life is categorized by interconnections and interactions
Chapter 2
Matter- Anything that occupies space and has mass
Element- A substance that can’t be broken down with any ordinary chemical means
Compound- Two or more elements combined in a fixed ratio
A compounds properties are different from the elements that make it up
Trace elements- elements that are essential to life but are found in minute quantities in the human body
Atom- The smallest unit of matter that still retains properties of elements and life
Sub-atomic particles- proton, neutron, electron
Nucleus- An atom’s core or center
Proton and neutron are found inside the nucleus and electrons are found in electron shells surrounding the nucleus
Proton- positive change
Electron- negative charge
Neutron- no charge
You can tell atoms apart by their atomic number.
Atomic number- number of protons
Atomic mass- proton + neutron
Isotopes- An atom having the same number of proton but different number of neutrons
Radioactive isotope- An isotope whose nucleus decays randomly giving off particles and energy. They can be helpful in dating fossils and diagnosing diseases
Electron shell- A level of electrons characterized by their distance from the nucleus
Atoms to make their electron shell full will:
Share electrons
Give up electrons
Accept electrons
Chemical bond- Atoms being held close together held by attraction
3 types of Chemical bonds:
Ionic bond
Transfer of electrons
attractions between oppositely charged ions
Covalent bond
Atoms sharing a pair of electrons
Strongest chemical bond
Atoms don’t always share the electrons equally
Can be single, double of triple bonds
Electronegativity- The measure of an atoms attraction for shared electrons
2 types of covalent bods:
A. Non-polar covalent bond- two atoms having the similar electronegativity and sharing electrons equally.
B. Polar covalent bond- two atoms having different electronegativity. causes partial charges. the atom that most of the shared electrons is partially negative while the atom the gets less of the shared electrons is partially positive.
Oxygen is one of the most electronegative elements.
Molecules- Atoms held together by covalent bonds
Hydrogen bonds
Weakest chemical bond
Formed between 2 individual water molecules between the partially negative region of the first molecule and the partially positive region of the second molecule
Chemical reaction- The making and breaking of chemical bonds leading to changes in the composition of matter
Reactant- The starting material in a chemical reaction
Product- The final material in a chemical reaction
Chemical reactions don’t create of destroy matter so they have to be balanced
Properties of water
Cohesion- When a water molecule sticks to another water molecule
Adhesion- When a water molecules sticks to other surfaces
Surface tension- The measure of how difficult it is to stretch or break the surface of a liquid. Water has high surface tension because of the hydrogen bond holding the molecules together
Water’s hydrogen bonds moderate temperature
Thermal energy- heat that comes from kinetic energy
Temperature- The measure of the average thermal energy
Heat- Thermal energy in transfer from one body of matter to another
Evaporative cooling- The process when the surface becomes cooler during evaporation. A result of molecules with the greatest energy changing from liquid to gas.
Ice floats because it is less dense than water
Water is less dense as a solid because of hydrogen bonds
The freezing of water make the hydrogen bonds distant making it decrease in density
Water is the solvent of life
Solution- A liquid consisting of a uniform mixture of two or more substances
Solvent- The dissolving agent is the solvent
Solute- substance that is dissolved is a solute
Aqueous solution- Aqueous solution is one in which water is the solvent.
In aqueous solutions some water will break into ions. those ions are the hydrogen ion(H+) and hydroxide ion(OH-).
The chemistry of life is sensitive to acidic and basic conditions
Acid-A substance that releases hydrogen ions to solutions. From 0-6.9 on the PH scale
Base-A substance that releases hydroxide ions to solutions. From 7.1-14 on the PH scale
PH scale-Used to describe how acidic or basic a solution is
PH scale increases by 10 at every level
Bluffers-chemical substances that minimize changes in pH
Chapter 20
An animals structure isn’t perfect, its just good enough to function
A structure of an ancestral organism can be modified to function in a descendant organism
Structure fits function at all levels of organization
Anatomy - The study of the form of an organism’s structure
Physiology - The study of the functions of those structures
Hierarchy of life
Cells - building block of life
Tissues - a group of cells that serve a similar functions
Organ - made up of 2 or more tissues to perform a specific task
Organ System - made up of multiple organs to perform on or more vital body function
Organism - made up of multiple organ systems each for different tasks
Types of tissues
Epithelial tissues - closely packed cells that cover organs and cavities
Helps as a protective barrier, secretion, exchanging materials and nutrient absorbtion
One side is attached to a dense mat of protein and the other side is what faces the outside and is aka Apical surface
They are organized by their cell shape and size
Ex: skin, tube in kidney, blood vessels, lining in intestines
Connective tissues - sparse population of cells scattered throughout a matrix
Form the framework of the bods, bind and support other tissues
Ex: blood, cartilage, bone
Muscle tissues - made up of long cells known as muscle fibers
Help with movement
Ex: skeletal muscle, cardiac muscle, smooth muscle
Nervous tissues - senses stimuli and rapidly transmits info
Also transits biological information
Main unit of the nervous tissue is the Neuron which is a tissue that is uniquely specialized to conduct electrical impulses
Ex: brain, spinal cord, nerves
Chapter 30
Respiratory and Circulatory Functions
Circulatory system - Heart and three types of blood vessels, arteries, veins and capillaries, Transportation network for blood
Circulation is maintained in the veins by the activity of skeletal muscles
Heart - A muscular pump that keeps blood moving to every part of the body
Arteries
Strong and flexible blood vessels that carry blood away from the heart to the rest of the body
Carry O2 rich blood
Smaller arteries (arterioles) connect arteries to capillaries
Veins
Larger diameter and thinner wall
Blood vessels that carry blood from the rest of the body to the heart
Carry O2 poor blood
Has valves to prevent blood from flowing backwards
Smaller veins (venues) connect the veins to capillaries
Capillaries
A system that connects arteries and veins
Only one cell thick
Easy for materials to diffuse into and out of them
Main function of Circulatory system
Transport blood and other materials
Bring supplies to cells
Carry cell’s waste
Keep O2 poor blood and O2 rich blood from mixing
Maintain body temperature by distributing/ conserving internal heat
Blood and blood pressure
Blood pressure - The measure of the force in which blood pushes against the artery wall
Systolic pressure - Measures pressure in the artery after the left ventricle has contracted, numerator
Diastolic pressure - Measures pressure after the left ventricle has relaxed, denominator
Blood - made up of plasma, red blood cells, white blood cells and platelets
Plasma
Mostly water and makes up 55% of blood
Contains proteins, amino acids, hormones, vitamins…
Movement of these materials helps maintain homeostasis
Contains proteins that help stabilize blood volume and control bleeding
Red blood cells
Transports O2 to cells and carry CO2 away from them
Binds to Hemoglobin that gives it its reddish color
Has protein markers that defines a persons blood type and rh factor
It is Important to know a person’s blood type and rh factor because if it isn’t the correct blood type the white blood cells will attack it which will cause the blood to clump
White blood cells
Defend the body against infections
Remove foreign materials and dead cells
Don’t contain hemoglobin
They are not limited to the circulatory system
Are considered part of the immune system
Platelets
Cell fragments that help control bleeding
Form a net around an injury and release clotting factors to stop bleeding and create a seal around a wound
Hemophilia- a genetic disorder, inability to create clotting factors
Circulatory diseases
They mainly affect the heart and arteries
Arteriosclerosis - Artery walls become thick and inflexive
Atherosclerosis - Blood flow is partially or fully blocked by plaque that is collected on the walls of arteries
Both diseases can lead to a stroke, heart attack or kidney damage
Hypertension - Permanently high blood pressure
Respiratory system - Nose/Mouth | trachea | bronchi | bronchioles | alveoli
Mechanics of Breathing - muscles of the ribcage and diaphragm
Inhale - low pressure in the lungs | diaphragm flattens and moves downward
Exhale - high pressure in the lungs | diaphragm relaxes and rises
Main function of Respiratory system
Exchange gases
Bring O2 into the body
Expel CO2 and water vapor
Both systems work together to maintain homeostasis
Main goal - increase surface area for gas exchange
Gas exchange
Alveoli and capillary is the only place where gas exchange with the atmosphere takes place.
Gas exchange of the lungs have three principles
O2 and CO2 are carried by the lungs
Gas moves by diffusion | from high to low concentration
Lining of the alveoli must be moist to her gasses diffuse
Capillaries surround alveoli
1. Blood in the capillaries have lower concentration of O2 compared to alveoli so O2 diffuses from the alveoli to the capillaries and binds with Hemoglobin
The blood in capillaries contain Red blood cells, a type of cells that picks up O2 from the lungs to the body cells
The O2 molecules in red blood cells bind to Hemoglobin, an iron rich protein that gives blood it’s reddish color
2. CO2 concentration in the blood is higher compared to alveoli so CO2 diffuses from capillaries to the alveoli
CO2 is expelled from the body with some water vapor
Gas exchange and the nervous system
Gas exchange is an automatic function regulated by the brainstem
These centers monitor dissolved gasses in the blood especially CO2 concentration
When u exercise the CO2 concentration increase which makes the blood acidic which makes sensors in the respiratory and circulatory system send signals to the brainstem
The brainstem send signals to stimulate the diaphragm and rib cage muscles to work harder
Respiratory Diseases
Damage to the respiratory system makes gas exchange more difficult
Emphysema
Caused mainly by smoking
Destroys the alveoli and reduces surface area for gas exchange
Has no cure
Asthma
Causes bronchioles to constrict due to muscle spasms
Makes it hard to move air in and out the lungs
Can be relieved by taking medicine
Cystic fibrosis
Genetic disease that causes the lungs to produce mucus
Mucus blocks airways and allows microorganisms to thrive in lungs
Causes lung infections
Treatment does exist
The heart and Circulation
The hear has four chambers
Atrium - Smaller chambers, receive blood into the heart, upper half | two sides left and right
Ventricles - Larger chambers, send blood away from the heart, lower half | two sides left and right
Septum - A thick wall of tissue that separates the left and right ventricles
Valves - The heart’s flaps that prevent blood from flowing backward, opens when the atria of ventricles contract and close when they relax
Heartbeat
The first heartbeat takes place in the atria and then in the ventricle
The first contraction starts at the SA node aka the pacemaker which make the atrium contract
The signal from the SA node spreads and stimulates the AV node which makes the ventricles contact
Blood flow in the heart
O2 poor blood enters the right atrium which contracts and sends blood into the right ventricle
The right ventricle contracts and sends blood into the pulmonary artery which carries it to the lungs for gas exchange
O2 rich blood returns to the heart through the pulmonary vein and enters the left atrium. Atrium contracts and sends blood to left ventricle
Left ventricle contracts and sends blood out of the heart to the rest of the body through the aorta
Types of Circulation
Pulmonary circulation- occurs between the heart and lungs, its main function is to carry O2 poor blood to the lungs and O2 rich blood to the heart
Systematic circulation- its main function is to carry O2 rich blood to body cells and O2 poor blood back to the heart
Homeostasis is maintained by matching respiration with the O2 needs of the body.
Chapter 3
Carbon
Carbon based molecules are called Organic compounds
Carbon’s valance is 4
Valance- An atoms need for electrons to be stable
Carbon can bond to four other atoms by sharing electrons
One of the simplest organic compound is Methane
Compounds only composed of Carbon and Hydrogen are called Hydrocarbons
Carbon Skeleton- a chain of carbon atoms
Carbon skeletons can:
vary in length
be branched or unbranched
have double bonds
be arranged in rings
Isomers- compounds with the same formula but different arrangements
Polymers- forms that are made from identical building blocks
Macromolecules- another word for polymer
Monomers- building blocks of polymers
Micromolecule- another word for monomer
Dehydration synthesis- process that linked up monomers to make polymers by removing water from the joint molecules
Hydrolysis- A process that separates polymers into different monomers by adding water into the compound
Enzymes- A substance used to speed up chemical reactions
Organic compounds
All life depends on the properties and reactions of the 4 organic compounds
Carbohydrates
Monomer- Monosaccharides(simple sugar)
Monosaccharides contain a hydroxyl group(-OH) and a carbonyl group(C=O)
Sugars dissolve in water because the hydroxyl group forms hydrogen bonds with water
Made up of Carbon, Hydrogen and Oxygen
Always in a 1:2:1 ratio
2 monosaccharides can form disaccharides like maltose and sucrose by a dehydration synthesis
In an aqueous solution sugars from ring like shapes
The bond created when sugars are joined together is called a Glycosidic linkage
Food sources
sugar
bread
pasta
cereal
Function
instant energy
can be used to make cellulose and parts of the cell membrane
stores energy as glycogen in animals and starch in plants
Polysaccharides
Are polymers composed of thousands of monosaccharides
Are usually Hydrophilic (water loving)
Function
Serve as storage molecules
Serve as structural compounds
Examples
Starch
Glycogen
Cellulose
Chitin- used by insects to build an exoskeleton
Lipids
Monomer- Glycerol and Fatty acids
Is hydrophobic (water fearing)
Contains twice the amount of energy as polysaccharides
Made up of Carbon, Hydrogen and Oxygen linked together by non polar covalent bonds
Function
Long term energy storage
Thermal regulation
Cushions vital organs
Types of Lipids
Fats- One glycerol linked to three fatty acid chains by dehydration synthesis
They are often called Triglycerides because of their structure
The bond between fatty acids and glycerol is called an Ester Bond
Fatty Acids- Can be Saturated or Unsaturated
Saturated- Dripping wet with hydrogen
No carbon carbon (C=C) bond
Solid in room temperature
Mostly animal fat like butter and red meat
Bad for a person’s health
Unsaturated- Forms a kink from its carbon carbon (C=C) bond
Liquid in room temperature
Mostly plant fat like corn and olive oils
Good for a person’s health
An unsaturated fat can be turned into a solid or semisolid by Hydrogenation
Hydrogenation creates Trans fats which are associated with health risks
Phospholipids- One glycerol attached to two fatty acid chains. The Glycerol is attached to a phosphate group.
The phosphate group and glycerol make up the Hydrophilic heads
The fatty acid chains make up the Hydrophobic tails
Phospholipids cluster into a bilayer of phospholipids
The hydrophilic heads are in contact with the water of the environment and the hydrophobic tails are in the center of the bilayer
Steroids- Lipids where the carbon skeleton contains four fused rings
Makes up our hormones
Cholesterol- A common component in animal cell membranes
Anabolic steroids- Synthetic variations s of testosterone that can cause buildup of muscle and bone mass.
Often prescribed to treat anemia
Abused by some athletes
Consequences include: violent mood swings, depression, liver damage, cancer etc
Protein
Monomer- Amino acids
Contains Carbon, Hydrogen, Oxygen and Nitrogen
Are involved in every dynamic function in our body
Amino Acids
Made up of - an animo group(H-N-H)
- a carboxyl group(O=C-OH). It makes amino acids acid
- A central carbon that is bonded to hydrogen and the R group
Amino acids always have an N-C-C structure
The R group gives amino acid its chemical properties
Amino acids are classified as either hydrophobic or hydrophilic
When two amino acids are being bonded the carboxyl group of one amino acid joins the animo group of the other amino acid form bonds known as Peptide bonds
More amino acids can be added to create a chain of Polypeptides
Dipeptide- two amino acids bonded by dehydration synthesis
The shape of a protein determines its function
The shape of a protein is caused by the amino acid sequence
If a shape of a protein is altered, it can no longer function
Denaturation- a process where a protein
unravels
loses its shape and
loses its function
Proteins can be denatured by:
Changes in salt concentration
Changes in PH
High heat
Function
Acts as an enzyme
Include antibodies of the immune system
Transmit signals to cells
Serve as a source of amino acids for developing embryos
Nucleic Acid
Monomer- Nucleotide
Consists of Carbon, Hydrogen, Oxygen, Nitrogen and Phosphorus
Nucleotides- have three parts
Sugar
Phosphate group
Nitrogen base
Nucleotides are the monomers of DNA and RNA
A nucleic acid polymer aka polynucleotide is formed by the dehydration synthesis that bonds the phosphate group of one nucleotide to the sugar of the next nucleotide.
This bond creates a sugar-phosphate backbone with protruding nitrogen bases
Function
store genetic Information
transmit genetic information
An amino acid sequence of polypeptides are programmed by a unit of inheritance called Genes
Genes- consists of DNA that is inherited from an organism’s parents
DNA
Provides directions for its own replication
programs a cell’s activities by directing proteins
DNA doesn’t build proteins directly
DNA→RNA→Protein
In DNA, the sugar that makes up the nucleotide is a five carbon sugar called deoxyribose
DNA’s nitrogen bases are
A
T
C
G
A→T
C→G
The letters in DNA that go together are called Base pairs
Two strands of polynucleotides that form a double helix
RNA
A single polynucleotide strand
In RNA, the sugar that makes up the nucleotide is a five carbon sugar called ribose
RNA’s nitrogen base has
A
C
G
U
Chapter 4
Cell- is the simplest collection of matter that is alive
They were first observed by Robert Hooke in 1665
Microscopes were developed for a clearer view of cells and cellular structures
There are 2 major types
Light microscopes
most frequently used microscopes
can magnify up to 100x
can’t provide the details of a small cell’s structure
can’t show organelles other than the nucleus
Is safer and keeps cells alive
Electron microscope
uses a beam of electrons
used to view the ultra structure of cells
can magnify up to 100,000 times
Cells are dead
2 types
Scanning electron microscope- used to scan and study cell surface
Transmission electron microscope- used to study internal cell structure
Magnification- The increase in the size of an object
Resolution- The measure of the clarity of an image
Cell theory
all living cells are composed of cells
all cells come from other cells
refutes the concept of spontaneous generation- life force in the air
Measurements
Most organelles are between 10-100nm
The external differences between eukaryotic and prokaryotic cells can be seen between 1-100µm
Cells must be able to
be large enough to hold DNA, protein and other structures
be small enough to allow a surface to volume ratio that allows enough exchange with the environment
* The smaller the cell the faster the rate of osmosis will be
Volume = (l)(w)(h)
Surface area = (l)(w)(number of sides)
Plasma membrane- forms a flexible boundary between a cell and its surrounding
Phospholipids form a two layer sheet called a Phospholipid bilayer that has:
hydrophilic heads that are exposed to the environment
hydrophobic tails that are inward shielded from water
The duality between the heads and tails controls what goes in and out
Membrane proteins- are either
attached to the membrane surface
embedded in the phospholipid bilayer
There are two types
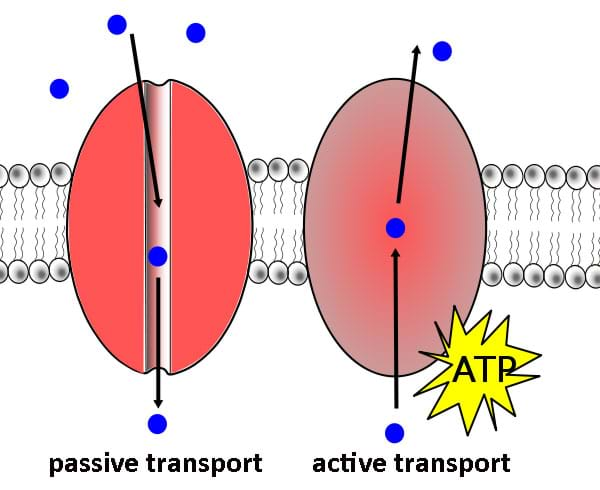
Passive transport- tunnels that shield ions and other hydrophilic molecules as they pass through the center of the bilayer
Active transport- serve as pumps that use energy to actively transport molecules in and out of the cell
There are two types of cells
Prokaryotic
No nucleus
Bacteria and Archaea
No true organelles
No membrane bound organelles
No mitochondria
Has a plasma membrane
Has ribosome
Has DNA chromosomes in the Nucleoid
The surface of prokaryotic cells may
be surrounded by a cell wall
have a capsule surrounding the cell wall
Have short projections called Fimbriae that help attach it to other cells
Have longer projections called Flagella that help the cell move efficiently
Eukaryotic cells
Has a nucleus
Plant, Animal, Fungi and Protista
Has membrane bond organelles
Has a mitochondria
Has a plasma membrane
Has a ribosome
Has DNA in the Nucleus
The structure of the organelles in eukaryotic cells have four basic functions
Genetic control and reproduction
Manufacture, distribution and breakdown of molecules
Energy processing
Structural support
The internal membranes of eukaryotic cells partition into compartments
Cellular metabolism- The many chemical activities of cells, occurs within organelles
Differences between plant and animal cells
Animal cells have lysosomes and plant cells don’t
Animal cells have centrioles and animal cells don’t
Plant cells have a mitochondria and chloroplast but animal cells only have a mitochondria
Plant cells have a rigid cell walls and animal cells don’t
Plant cells have chloroplast and animal cells don’t
Plant cells have a central vacuole and animal cells don’t
Organelles and their functions
Nucleus
DNA replication
RNA synthesis
Assembly of ribosomal subunits
Ribosomes
Protein synthesis
Rough ER
Lipid and protein synthesis
Synthesis of secretory proteins
Synthesis of hydrolytic enzymes
Formation of transport vesicles
Smooth ER
Lipid synthesis
Detoxification(in liver cells)
Calcium ion storage(in muscle cells)
Golgi apparatus
Modification and sorting of ER products
Formation of lysosomes and transport vesicles
Lysosomes
(found in some protists and animal cells)
Digestion of ingested foods
Recycling of a cell’s damaged organelles
Vacuoles
Storage of food
Storage of waste products for removal
Peroxisomes
Detoxify alcohol(in liver cells)
Cholesterol synthesis
Breakdown hydrogen peroxide
Mitochondria
Conversion of chemical energy from food to the chemical energy of ATP (Cellular respiration)
Chloroplast
Conversion of light energy to the chemical energy of sugars (Photosynthesis)
Cytoskeleton
(microfilaments, intermediate filaments and microtubules)
Maintenance of cell shape
Supports plasma membrane and other organelles
Helps in cell movement ex: movement of the cilia and flagella
Plasma membrane(Cell membrane)
Regulate what goes in and out of the cell
Extracellular matrix(animal only)
Support and regulate cellular activities
Cell junctions
Help in communication between cell
Helps in binding of cells in tissues
Cell wall(plant only)
Support and protect cells
Helps in binding of cells in tissues
*In-depth descriptions of organelles
*The Nucleus, it’s parts and the Ribosome can be grouped as the Genetic control group
Nucleus
It contains most of the cell’s DNA
Controls cellular activities
Also known as “the cell’s genetic control center”
Directs protein synthesis by making mRNA(Messenger RNA)
Chromosomes- Protein structures associated with DNA
Nuclear envelope-
Double membrane
Has pores to let materials flow in and out the nucleus
Attached to the Endoplasmic Reticulum(ER)
Chromatin-
Mixture of DNA and proteins
Used to package DNA into small capsules so that it fits in the nucleus
Nucleolus-
An important structure in the nucleus
The place where ribosomal (rRNA) synthesis takes place
Ribosomes
Involved in protein synthesis
Synthesized from rRNA produced in the nucleolus
Large amount of protein synthesis = large number of proteins
There are two types
Free ribosomes
Suspended in the cytoplasm
Involved in making proteins that function within the cytoplasm
Bound ribosomes
Attached to the ER
Associated with the nuclear envelope
Associated with proteins packed in organelle or exported form the cell
Cytoplasm- A liquid found in cells that is bound by the cell membrane and made up of water, enzymes, organelles and salts.
The Endocrine System
Most of Eukaryotic cell’s organelles are a part of the endocrine system
All the organelles are made up of a phospholipid bilayer
Some of them are physical connected and others are related by the transfer of membrane segments by vesicles
Many of these organelles work together in the synthesis, storage and export of molecules
Includes:
Nuclear Envelope
Endoplasmic reticulum (ER)
Golgi apparatus
Lysosomes
Vacuoles
Plasma membrane
Vesicle- small sacs that are made of membrane
Endoplasmic Reticulum(ER)
Two types:
Smooth ER:
Lacks attached ribosomes
Produces enzymes important in lipid, oil, phospholipid and steroid synthesis
Produces enzymes that process drugs and alcohol
Helps store calcium ions
Rough ER:
Has ribosomes attached to it
Site of protein synthesis
Lines the outer layer of membranes
Produces additional membranes for itself
Produces proteins used for secretion
Functions in protein folding, sorting and transporting to their destinations
Smooth and Rough ER are physically connected
Golgi apparatus
Functions as the finishing factory for products made in the ER
Products travel From the ER to the Golgi apparatus through vesicles
One side of the Golgi apparatus acts as a receiver and the other as a sender
Products are modified as they go from one side to the other then they travel through vesicles to other sites
Lysosomes
The digestive compartments within a cell
Enzymes and membranes are produced in the ER→ goes to the Golgi apparatus for further processing→ lysosomes separate the Important enzymes from the rest of the cell
Help digest food engulfed by the cell
Food vacuole binds with lysosome→ Enzymes in lysosome digests it→ nutrients are released into cell(cytosol)
Help remove or recycle damaged parts of a cell
Damaged organelle gets enclosed in a vesicle→ lysosome fuses with the vesicle→ lysosome dismantles its content and breaks it down
Lysosomal Storage Disease
Taysach disease
Lysosomal disorder
Can be inherited
Leads to non functioning lysosomes
Often seen in infants
Unavoidable death
Vacuoles
Large vesicles that vary in function
Function in protists → Eliminate water from protist
Function in plants → Digestive function, contain pigment, contain poisons that protect the plant
The function of vacuoles can be generalized as food and waste storage
Energy converting group
Mitochondria
Carry out cellular respiration in eukaryotic cells
Has two internal compartments
Inter-membrane space- The narrow region between the inner and outer membranes
Mitochondrial matrix-
Contains the DNA of the mitochondria
Contains ribosomes
Contains enzymes that catalyze some of the reactions in cellular respiration
Has Cristae- The folds in the inner- membrane
Cellular respiration- Conversion of the chemical energy from foods to the chemical energy of ATP
Chloroplast
Photosynthesizing organelles in all photosynthesizing eukaryotes
Portioned into compartments
Thin inter-membrane space between the outer and inner membrane
Inside the Inner-membrane there is:
Stroma- A thick fluid that contains chloroplast DNA, ribosomes and many enzymes
Thylakoids- A network of connected sacs
Granum- A stack of connected thylakoids, the place where chlorophyll molecules trap solar energy
Endosymbiosis-
The mitochondria and chloroplast have DNA and ribosomes unlike the other organelles
The Structure of the DNA and ribosomes are very similar to that of prokaryotic cells
Endosymbiosis means one prokaryote being engulfed by another prokaryote for the mutual benefit of both prokaryotes
Endosymbiont theory- A theory that states that Mitochondria and chloroplast were once small prokaryotes and that they began living in larger cells
Cytoskeleton group
Cytoskeleton- A network of protein fibers that function in structural support and movement
Movement and cellular regulation result in the cytoskeleton interacting with proteins Called Motor proteins
Cytoskeleton is composed of three kinds of fibers
Microfilament- Support the cells shape and are involved in movement
Intermediate filament- Reinforce that cells shape and anchors organelles
Microtubules- Provide the inability the cell to be bent or forced out of shape and serve as tacks in organelle movement
Cilia and Flagella
Protists have a flagella and cilia that are important in movement but other multicellular organisms have them of different reasons
Cells that sweep mucus of of the lung have cilia
Animal sperms have a flagella
Flagellum are longer than cilia and move in a whiplike motion
Cilia moves in rowing motion
Both cilia and flagella are made of microtubules that are wrapped in an extension of the plasma membrane
A ring of nine microtubule doubles surround the central pair of microtubules in a 9+2 pattern
Both cilia and flagella move by bending motor proteins called Dynein feet
The feet attach and exert a sliding force on an adjacent doublet
The arms then releases and reattach a little further along and the process is repeated
This causes the microtubules to bend
Extracellular matrix(only in animal cells)
Helps hold tissued together
Protect and support the plasma membrane
It is attached to a cell through intergrins- glycoproteins that bind to membrane proteins
Intergins span the plasma membrane and connect to the microfilaments of the cytoskeleton
Cell junctions
Helps adjacent cells communicate and interact
Three types(only in animal cells)
Tight junctions- Prevent leakage of extracellular fluid across a layer of epithelial cells
Anchoring junctions- Fastens cells together into sheets
Gap junctions- Channels that allow molecules to flow between cells
Plasmodesmata(only in plant cells)- Serves in communication between plant cells
Cell wall(only in plant cells)
Protects and provides skeletal support
Keeps the cell upright against gravity
Primarily composed of cellulose
Chapter 5
Bioluminescence- the process where organisms use energy converting reactions to produce light
Used by marine animals to hide from predators
Many of the cell’s reactions take place in the organelles and use membrane embedded in the membranes of these organelles
5.1
The cell membrane is constructed in a structure called fluid mosaic
The cell membrane is called a fluid mosaic because its components float in a cytoplasmic fluid
Membranes are made of:
A phospholipid bilayer
Proteins
Glycoprotein
Transport protein
Carrier protein
Channel protein
Surface protein
Cholesterol (embedded)
Cytoskeleton(on the inside of the cell)
Extra cellular matrix(on the outside of the cell)
Intercellular junction
Lipids
Cholesterol
In animal cell membranes they help:
Stabilize membranes at warmer temperatures
keep the membrane fluid at a lower temperatures
Proteins
Membrane proteins have many functions like
Help maintain cell shape and coordinate changes inside and outside the cell
Act as receptors for chemical messages from other cells
Function as enzymes
Help in cell-cell recognition, ie glycoproteins
Help in intercellular junctions that attach adjacent cells together
Transport molecules in and out of the cell
Glycoprotein
In membranes they are used to:
Cell-cell recognition- recognize other cells as familiar or foreign
Cell adhesion- help cells attach to other cells
Surface proteins
function in communication between a cell and its environment
Membranes are selectively permeable- allow some substances to cross more easily that others
5.2
Phospholipids
Are made up of:
A polar phosphate head
2 non-polar fatty acids chains that are unsaturated and have kinks
The kinks prevent phospholipids from packing tightly together and keeps them in liquid form
Phospholipids are the key components in cell membranes
Phospholipids spontaneously self-assemble into simple membranes because of their duality
The formation of membrane enclosed collections of molecules was a critical step in the evolution of the first cells
5.3
Passive transport- transportation of molecules that doesn’t require energy.
Diffusion- the tendency for particles to move from areas of high concentration to areas of low concentration
Diffusion is a type of passive transport
Concentration gradient- the difference in concentration of a substance for one point to another
*During diffusion molecules move down their concentration gradient until they reach equilibrium
The original kinetic energy from molecule’s constant random motion causes them to diffuse
Water is one of the most important substances that crosses the cell membrane
Osmosis- the diffusion of water across a selectively permeable membrane
Osmosis is a type of passive transport
*if a membrane is permeable to water but not a solute and separates the solutions with different solute to solvent ratio
Water will move across the membrane and move down its concentration gradient
Move until the solute concentration(solute to water ratio) on both sides is equal
5.5
Tonicity- the ability of a solution to cause a cell to gain or lose water
tonicity depends on the concentration of a solute on both sides of the membrane
Types of solutions
Hypertonic solution- High concentration of solute. Low concentration of water.
Water needs to enter to make in isotonic
Isotonic solution- Equal concentration of solute and solvent. water enter and exits in the same rate.
Hypotonic solution- Low concentration of solute. High concentration of water.
Water needs to go out to make it isotonic
Plants and Animal cells in different types of solutions
Animal cells in an isotonic solution- cell volume stays the same cuz water molecules enter and exit in the same rate
Animal cells in a hypotonic solution- cells swell and burst lysis cuz water enters quicker than it exits
Animal cells in a hypertonic solution- cell shrivel and dies crenate cuz water exits quicker than it enters
Plant cells in an isotonic reaction- cell volume will decrease but won’t die flaccid
Plant cells in a hypotonic solution- cell will swell but not burst turgid/ normal b/c of their rigid cell wall
Plant cells in a hypertonic solution- cell will shrivel and die plasmolyze
For animal cells to survive in a hypertonic or hypotonic environment they engage in osmoregulation- the regulation of the movement of water into and out of the cell
EX: the contractile vacuole in a paramecium
Plant cells, prokaryotic cells and fungi have their own adaptation of osmoregulation:
Hypotonic environment- the cell wall of these cells exerts pressure that prevents the cells from taking too much water and bursting
Hypertonic environment- cells will shrivel(includes animal cells)
5.6
Molecules that can easily diffuse across the cell membrane
hydrophobic/ non-polar substances
small molecules like O2 and CO2
small lipids
Molecules that don’t easily cross the cell membrane
hydrophilic/ polar substances
charged molecules ie ions
Facilitated diffusion- transport proteins helping move molecules that can’t diffuse easily
It doesn’t require energy
Relies on the concentration gradient
Transport proteins help in facilitating diffusion by:
becoming a tunnel for ions aka Channel protein ,only transports water soluble things
binding to the molecules and shooting them out on the other side aka Carrier protein , transports water soluble and insoluble things
In both of these situations the protein is specific to their substrate just like enzymes
*Water is polar so its diffusion thorough the membrane is slow
Aquaporin- a very rapid diffusion of water that’s made possible by a protein channel
5.7
Aquaporins were discovered by Dr. Peter Agre
His research on the Rh protein used in blood typing led to this discovery
5.8
In active transport a cell
uses energy in the form of ATP
moves a solute against its concentration gradient
In active transport the transport proteins have specific solutes that they can pump
EX: the sodium potassium pump
Steps for active transport
Solute binds with the transport protein
ATP phosphorylates the protein and ATP becomes ADP
Protein changes shapes and shoots solute on the other side
Protein reverses shape and dephosphorylates
5.9
A cell has two mechanisms to move large molecules like proteins and carbs across the cell membrane
Exocytosis- used to export bulky molecules
Ex: Cells exporting protein
Steps in exocytosis
A. Molecules inside the vesicle move towards the cell membrane
B. The vesicle docks on the membrane
C. The vesicle fuses with the cell membrane and releases the molecules out
Endocytosis- used to import substances that are useful to the cell
Ex: A prokaryotic cell taking in a mitochondria
Steps in endocytosis
A. Cell membrane makes a pocket filled with fluid for the molecule to come
B. Molecules come into the pocket and the membrane folds inwards
C. the membranes connect forming a vesicle or vacuole then moves into the cell
Endocytosis and exocytosis are both active transports but they don’t require a protein
Endocytosis and exocytosis both change the shape of the cell membrane for a short period of time before the phospholipids come back together because of their duality
Types of Endocytosis
Phagocytosis- engulfement of a particle by forming a vacuole:
AKA cell eating
also used for digesting waste
Pinocytosis- engulfment of fluids by forming a vesicle
Aka cell drinking
Receptor-medicated endocytosis- used receptors in a receptor coated pit to interact with a specific protein and form a vesicle
5.10
Cells- small units that house chemical reactions.
Cells use chemical reactions for
cell maintenance
creation of cellular parts
cell replication
A cell can’t be a cell without chemical reactions
Energy- the capacity to cause change or to perform work
There are two kinds of energy
Kinetic energy- the energy of motion
Potential energy- the energy of location or structure
In bio potential energy is found in
the arrangement of atoms in molecules
the covalent bonds that holds molecules together
Making and breaking these bonds release the potential energy
Heat or thermal energy- a type of kinetic energy associated with random movements of atoms or molecules
Light- a type of kinetic energy that can be harnessed from the sun and be used to power photosynthesis
Law of conservation of energy- energy can’t be created nor destroyed
Chemical energy- the potential energy(found in the covalent bonds of a molecule) that is available for release in a chemical reaction
It is the most important type of energy for living organisms to power the work of cells
Thermodynamics- the study of energy transformations that occur in a collection of matter
Scientists use the words
System - what they study. ex: a single cell
Surroundings- the environment of the system. ex: blood stream
Laws of thermodynamics
First law- energy in the universe is constant aka can’t be created or destroyed
Second law- energy conversions increase the disorder of the universe
Entropy- the measure of disorder or randomness
*The universe leans towards disorder
*Humans are endothermic and endergonic
Cellular respiration- The transfer of energy from the chemical energy of the food we eat and the oxygen we breathe to the chemical energy of ATP.
This reaction is very controlled and releases energy slowly
In this reaction the oxygen we breath is used as a key component
5.11
Types of chemical reactions
Exergonic-
Releases the energy in Covalent bonds of the reactants
Easier for cells to accomplish compared to endergonic reaction
Downhill reaction
Spontaneous
EX: Burning wood, Cellular respiration, hydrolysis
Macromolecules → Monomers
Endergonic-
Requiere a constant input of energy
Uphill reaction
Not spontaneous
EX: dehydration synthesis, protein, carb, nucleic, lipid synthesis and photosynthesis
*All chemical reactions require:
An enzyme
ATP
Addition or removal of water
Metabolism- The total number of an organisms chemical reaction
Metabolic pathway- A series of chemical reactions that
Builds a complex molecule or
Breaks down a complex molecule
Energy coupling- Uses the energy released in exergonic reactions to fuel endergonic reactions.
Usually uses the energy stored in ATP molecules
5.12
ATP(Adenosine triphosphate)-
Powers all terms of cellular work.
Is renewable source of energy
Energy form an exergonic reaction goes into an endergonic reaction to produce ATP
ATP consists of:
Nitrogenous base Adenine
Five carbon sugar Ribose
Three phosphate groups
Phosphorylation- A hydrolysis reaction that releases energy by transferring its third phosphate group to some other molecule
Most cellular work depends on ATP energizing molecules by phosphorylating them
* There are three main types of cellular work
Chemical
Mechanical
Transport
ATP drives all three of them
ADP + P → ATP
ATP gives away the last phosphate group and becomes ADP
ADP + P → ATP
5.13
Although biological molecules contain a lot of potential energy, It is not released spontaneously
An energy barrier must be overcome before a chemical reaction can begin called Activation Energy
Activation energy is the energy needed for a reactant molecule to move up hill to a higher but unstable energy before the rest of the reaction happens
One way to speed up a reaction is Adding heat but that kills our cells
Enzymes
Organic catalysts that are safe and can be used in living organisms to speed up a chemical reaction.
Reduces the Activation energy barrier
Increases the rate of the reaction without being consumed into the reaction
Usually proteins and sometimes RNA molecules
Does not add or remove energy of the final product
Very selective and has a shape that determines the enzyme specifically
Enzymes are specific because their active site fits only specific types of substrate
Substrate- The specific reactant to an enzyme
Active site- The space where the enzyme and substrate connect
5.14
Every enzyme has optimal conditions where its most effective
Most human enzymes work best at 35-40*C
Denaturation- happens when the PH, Salinity or Temperature is too high. It changes the shape of an enzyme making it not function
Most enzymes require a Non-protein helpers called cofactors
Cofactors-
Binds to the active site and functions in catalysis
Some are inorganic like zinc, iron and copper
Coenzyme-
An organic cofactor
Always vitamins
5.15
Inhibitor-
A chemical that interferes with an enzymes activity
Important in regulating cell metabolism
Enzyme inhibition- An inhibitor that prevents the enzyme from doing its work
Competitive inihibitor
Block the substrate from entering an enzymes active site
Reduces an enzyme’s productivity
Noncompetitive inhibitor-
Binds to an enzyme somewhere other that the active site
Changes the shape of the active site
Prevents the substate from binding
*Both competitive and non competitive prevent the substrate from bonding with the enzyme
Feedback inhibition- When the product acts as an inhibitor of one of the enzyme’s in the pathway that produced it
5.16
Many beneficial drugs act as enzyme inhibitors including
Ibuprofen- inhibits the production of prostaglandins
Blood pressure medicines
Antidepressants
Antibiotics
Protease- inhibitors used to fight HIV
Enzyme inhibitors have also been developed as pesticides and deadly poisons for chemical warfare
Chapter 6
Life requires energy
Energy ultimately comes from the sun
Cellular respiration takes place in mitochondria and photosynthesis takes place in mitochondria
Respiration- an exchange of gases aka breathing
Cellular respiration- the Aerobic- oxygen requiring harvesting of energy from food molecule
Cellular respiration is an exergonic reaction that transfers energy from the bonds in glucose to form ATP
Each glucose molecule produces 32 ATP molecules
Other organic molecule can also be used as the energy source
C6H12O6 + O2 → 6CO2 + 6H2O + ATP(heat)
The human body uses energy from ATP to fuel all its activities
Kilocalorie(kcal) - the quantity of heat required to raise the temperature of 1 kg of water by 1 degree celsius
The energy needed for life can be found in the arrangement of electrons in the chemical bonds that hold organic molecules together
When the Carbon-hydrogen bonds in glucose are broken electrons get transferred to oxygen because of its tendency to attract electrons
Energy can be released from glucose by simply burning it but the energy dissipated as heat and light isn’t available to living organisms
Cellular respiration is the controlled breakdown of organic molecules
Energy is:
a. gradually released in small amounts
b. captured by a biological system and
c. stored in ATP
Redox reaction(oxidation-reduction)- the movement of electrons from one molecule to another.
Oxidation- the loss of electrons
Reduction- the addition of electrons to one substance
In cellular respiration:
Glucose loses its hydrogens and becomes oxidized to CO2
Oxygen gains hydrogen atoms and becomes reduced to H2O
Enzymes are necessary to oxidize glucose and other foods
NAD+
important enzymes in oxidizing glucose
accepts electrons and
becomes reduced to NADH
There are other electron carrier molecules that function like NAD+
They form a staircase where the electrons pass from one to the next down the staircase. They are called Electron transport chain
As electrons are moved down the staircase ATP is generated
Cellular respiration can be divided into 3 stages
Glycolysis
Pyruvate oxidation and citric acid cycle
Oxidative phosphorylation
1.Glycolysis
occurs in the cytoplasm
begins cellular respiration
breaks down glucose into two molecules of 3 carbon compound called pyruvate
Releases 2 ATP molecules
Glucose→ 2 pyruvate
NAD+ → NADH
FAD+→ FADH2
2.Pyruvate oxidation and citric acid cycle/ krebs cycle
occurs in the mitochondria
oxidizes pyruvate into 2 carbon compounds
Supplies the third step with electrons
Releases 2 ATP molecules
2 pyruvate → CO2
NAD+ → NADH
FAD+ → FAD2
3.Oxidative phosphorylation
Occurs in the mitochondria
Uses O2 to phosphorylate ADP → ATP
Involves the electron transport chain and Chemiosmosis- Generating ATP through oxidative phosphorylation
Releases 28 ATP molecules
NADH + FADH2 + O2 → H2O + ATP
Aerobic- requires O2, glycolysis→ citric acid cycle→ Oxidative phosphorylation
Anaerobic- doesn’t require O2, glycolysis→ fermentation
Fermentation- a way of harvesting chemical energy that doesn’t require oxygen (Anaerobic). Main function is to oxidize NADH back to NAD+
Fermentation
takes advantage if glycolysis
Produces 2 ATP molecules for every glucose molecule
reduces NAD+ to NADH
The trick of fermentation is to provide an anaerobic path for recycling NADH back to NAD+
Your muscle cells and certain bacteria can oxidize NADH through Lactic acid fermentation.
Lactic acid fermentation:
NADH is oxidized to NAD+
Pyruvate is reduced to lactate
Lactate is carried by the blood to the liver where it’s converted back to pyruvate and oxidized in the mitochondria of liver cells
The dairy industry uses lactic acid fermentation by bacteria to male cheese and yogurt
Other microbial fermentation turn
Soybeans→ soy sauce
Cabbage→ sauerkraut
The baking and winemaking industries use Alcohol fermentation to harvest chemical energy
In this process Yeast( single celled fungi)
Oxidize NADH back to NAD+
Convert pyruvate to CO2 and ethanol
Obligate anaerobes vs. Facultative anaerobes
Obligate anaerobes:
Are poisoned by oxygen requiring anaerobic conditions
Live in stagnant ponds and deep soils
Facultative anaerobes:
Includes yeasts and many other bacterias
Can make ATP by fermentation or Oxidative phosphorylation
Chapter 7
Plants, algae and certain prokaryotes convert light energy to chemical energy and store it in sugar
Autotrophs:
make their own food through the process of photosynthesis.
sustain themselves
don’t usually consume organic molecules from other organisms
Photoautotrophs- use the energy of light to produce organic molecules
Chemoautotrophs- prokaryotes that use inorganic molecules as their energy source
Heterotrophs- are consumers that feed on plants, animals. they decompose organic material
Photosynthesis in plants:
takes place in chloroplast
converts CO2 and water into organic molecules
releases O2
Chloroplast are the major sites of photosynthesis in green plants
Chlorophyll
and important light absorbing pigment in chloroplast
makes plants green
plays a major role in converting solar energy to chemical energy
Chloroplast are concentrated in the cells of the mesophyll- the green tissue in the interior of the leaf
stomata- tiny pores in the leaf that allow the CO2 to move in and O2 to exit
Veins in the leaf deliver H2O absorbed by the roots
Chloroplast consists of an envelope of two membranes that:
enclose the inner compartment with a thick fluid called stroma
contain a system of interconnected membranous sacs called thylakoids
Thylakoids
are often concentrated into stacks called grana
have an internal space called thylakoid space
thylakoid membrane also have most of the machinery that converts light energy to chemical energy
Chlorophyll molecules are:
built into the thylakoid membrane
capture light energy
Photosynthesis is a redox (oxidation- reduction) process
CO2 becomes reduced to sugar as electrons from H2O are added to it
Water molecules are oxidized when they lose electrons along with hydrogen ions
6CO2 + 6H2O → C6H12O6 + 6O2
In photosynthesis
light energy is captured by chlorophyll molecules to boost the energy of electrons
light energy is converted to chemo energy
chemical energy is stored in the chemical bonds of sugars
Photosynthesis occurs in two stages
Light reactions- occurs i. the thylakoid membranes
H2O→ O2
ADP + P → ATP
NADP+ → NADPH by using light to excite the electrons
the NADPH produced by the light reactions provides the electrons for reducing carbon in the carbon cycle
Calvin cycle- occurs in the stroma of the chloroplast
Uses CO2 and ATP to assemble sugar molecules
Carbon fixation- a process that incorporates CO2 into organic compounds
After carbon fixation enzymes of this cycle make sugars by further reducing the carbon compounds
AKA light independent reaction
Sunlight contains energy called electromagnetic energy
Visible light is only a small part of the electromagnetic spectrum
Electromagnetic energy travels in waves
Wave length- the distance between the crests of two adjacent waves
Light behaves as discrete packets of energy called photons
Photon- a fixed quantity of light energy
*the shorter the wavelength the greater the energy
Pigments absorb light and are built into the thylakoid membrane
Plant pigments absorb some wavelengths of light and reflect or transmit others
*we see the color of wavelengths that are transmitted
Chloroplast contains several different pigments that absorb different wavelengths
chlorophyll a - absorbs blue, violet, red and reflects green
chlorophyll b - absorbs blue and orange and reflects yellow and green
carotenoids -
broaden the spectrum of colors used for photosynthesis
provide photoprotection- absorbing or getting rid of excess light energy that would have damaged the chlorophyll or interacted with O2 to create reactive oxidative molecules
Most plants use CO2 directly from the air and carbon fixation occurs when the enzyme rubisco adds CO2 to RuBP. These plants are called C3 plants.
they are called C3 plants because the first product of carbon fixation is a 3 carbon compound, 3- PGA
in hot and dry weather C3 plants:
close their stomata to reduce water loss
prevent CO2 from entering the leaf and O2 from leaving
as O2 builds up in a leaf, rubisco adds O2 instead of CO2 to RuBP, and a two-carbon product of this reaction is then broken down in the cell. This process is called Photorespiration because it occurs in the light, consumes O2 and releases CO2
Photorespiration uses ATP instead of producing it
C4 plants evolved in the means of:
carbon fixation that saves water during photosynthesis and
optimizes the Calvin cycle
C4 plants are called C4 plants because they fix CO2 into a four carbon compound
In hot and dry weather C4 plants keep their stomata mostly closed to conserve water
CAM plants- plants like pineapples and cacti
Conserve water by opening their stomata and admitting CO2 only at night
CO2 is fixed into a four carbon compound that:
banks CO2 at night and
released it into the Calvin cycle during the day
Chapter 8
A key characteristic of life is reproduction
Cell division
reproduction at the cellular level
duplicates the chromosomes and sorts the new sets into daughter cells
Cell division is used for:
reproduction of unicellular organisms
growth and development of multicellular organisms
replacement and repair of cells
sperm and egg production
There are two methods of reproduction
Asexual reproduction
produces offsprings that are identical to the original organism
involves the inheritance of all genes from one parent
Sexual reproduction
produces offsprings that are similar to the parents but vary in traits
involves the inheritance of unique sets of genes from two parents
Prokaryotes aka bacteria and archaea reproduce by binary fission
Cell division of prokaryotic cells is faster that cell division in eukaryotic cells
The chromosome of a prokaryote is
a single circular DNA molecule associated with proteins
a lot smaller that eukaryotic chromosome
Stages of binary fission in prokaryotic cells
duplication of the chromosome and separation of the copies
elongation of the cell and movement of the copies
division into daughter cells
Eukaryotic cells
are larger and more complex compared to prokaryotic cells
have more genes
store most of their genes on multiple chromosomes inside the nucleus
Eukaryotic chromosomes are made of Chromatin consisting of:
one long DNA molecule
Proteins that help maintain its structure and control the activity of the genes
When preparing for division the Chromatid becomes compact and visible under a light microscope
Before eukaryotic cell division the cell duplicates its chromosome making:
two copies called sister chromatids
joined at the waist called the centromere
When the cells divide the sister chromatids
separate from each other now called chromosomes and
sort into separate daughter cells
Cell cycle- an ordered sequence of events that extends from a cells that’s first formed from dividing until its own division
The cell cycle consists of two stages:
Interphase - duplication of cell contents
G1 - growth, increases the cytoplasm
S - duplication of chromosomes
G2 - growth, preparing for division
Mitotic phase - division
Mitosis - division of the nucleus
Cytokinesis - division of the cytoplasm
Mitosis occurs in a series of stages:
Prophase
Pro-metaphase
Metaphase
Anaphase
Telophase
Cytokinesis occurs at the same time as telophase
Prophase
a. Chromatin fibers become more tightly coiled and folded, forming discrete chromosomes that can be seen with the light microscope
b. The duplicated chromosomes appear as two sister chromatids
c. The mitotic spindle begins to form as microtubules rapidly grow out from the centrosomes
Pro-metaphase
a. The nuclear envelope breaks and disappears
b. Microtubules from the centrosomes extend and reach the chromosomes
c. Some of the microtubules attach to the kinetochore- a protein structure within the centromere
d. Other microtubules make contact with the microtubules coming from the opposite pole
Metaphase
a. At this phase the mitotic spindle is fully formed
b. The chromosomes line up on the metaphase plate- an imaginary plane equidistant between the two poles of the spindle
c. The centromeres of all chromosomes are lined up on the plate. For each chromosome, the kinetochores of the two sister chromatids are attached to microtubules from opposite poles
Anaphase
a. The sister chromatids separate at the centromere
b. Daughter chromosomes are moved to opposite poles
c. The cell elongates
Telophase
a. The elongation of the cell continues
b. The nuclear envelopes form around the now separated chromosomes
Basically reverse prophase
c. The chromatin fibers uncoil and the mitotic spindle disappears
Cytokinesis
a. The cytoplasm is divided into separate cells
Cytokinesis differs in animal and plant cells
Cytokinesis in animal cells
A Cleavage furrow forms a contracting ring of microfilaments, interacting with myosin, and
The cleavage furrow deepens to separate the contents into two cells
Cytokinesis in plant cells
A Cell plate forms in the middle, form vesicles containing cell wall material
The cell plate grows outward to reach the edges, dividing the contents into two cells
Each cell now possesses a plasma membrane and a cell wall
The cells within an organism’s body divide and develop at different rates
The rate of cell division is determined on what the cell does for the body
Cell division is controlled by:
The presence of essential nutrients
Growth factors
Density- dependent inhibition
Anchorage dependence
Growth factors - proteins that are released by a near by cell and stimulate cell division when taken in by a cell
Density- dependent inhibition - Cells divide until the presence on the cell touching shuts down cell division
Anchorage dependence - The need for cells to be in contact with a solid surface to divide. In humans its the bloodstream
The Cell cycle control system is a cycling set of molecules in the cell that
triggers and
coordinates key events in the cell cycle
Checkpoints in the cell cycle can
stop an event
signal on event to proceed
There are 3 major checkpoints in the cell cycle
G1 checkpoint
allows entry to the S phase or
Causes the cell to leave the cycle, entering a non dividing G0 phase
G2 checkpoint
M checkpoint
Cells in G0 maybe injured and won’t divide ever again
Cancer currently claims the lives of 20% of the people in the US
Cancer cells escape controls on the cell cycle
Cancer cells:
divide rapidly, often in the absence of growth factors
spread to other tissues through the circulatory system
grow without being inhibited by other cells
Cancer cells violate all of the cell cycle control system
Tumor - an abnormally growing mass of body cells. There are 2 types
Benign tumor - remain at the original site. Can be removed by surgery
Malignant tumor - spread to other locations called Metastasis
Metastasis- The spreading of cancer cells from their origin to other parts of the body. The newly spread cancer is identical in genetic makeup to the original cancer cell
The bloodstream/ circulatory system and the Lymphatic system/ immune system touches every cell in the human body.
Cancers are named are the organ/tissue it comes from
Carcinomas - arise in external or internal body coverings
Sarcomas - arise in supportive and connective tissue
Leukemias and Lymphomas - arise from blood- forming tissues
Localized tumors can be:
removed surgically
treated with concentrated beams of high-energy radiation
Chemotherapy is used for metastatic tumors
Cancer cells have the ability to release molecules that command the cells in the bloodstream to move towards them and feed them when cancer cells aren’t in direct contact with the bloodstream
In humans Somatic cells - body cells, have:
22 pairs of homologous chromosomes known as Autosomes that are the same size and genetic makeup
Sex chromosomes- X and Y chromosomes that differ in size and genetic makeup
XX → Female
XY → Male
Homologous chromosomes are matched in:
length
centromere position
gene locations
Homologous chromosomes have the same gene position but not the same allele
Locus- the position of a gene
A pair of homologous chromosomes are called a Tetrad
An organisms life cycle is a sequence of stages leading from the adults of one generation to the adults of the next
Humans and many animals and plants are Diploids with body cells that have
two sets of chromosomes
one from each parent
Ploidy- the number of copies of the genome
Meiosis is a process that converts a diploid into haploid
Diploid cells - have two homologous sets of chromosomes
Haploid cells - have one set of chromosomes NO HOMOLOGOUS SETS
Meiosis occurs in sex organs producing gametes- sperm and egg cells
Fertilization- the union of sperm and egg cells. forms a zygote. Occurs in the Fallopian tube
Zygote- has a diploid chromosome number one set from each parent
Meiosis has to happen to offset fertilization
All sexual life cycles alternate between a diploid stage and a haploid stage
In meiosis the cell goes from a diploid → haploid with sister chromatids → haploid with one set of chromosome
Meiosis has one cycle of duplication and two cycles of cell division
Stages of Meiosis
Meiosis 1
Prophase 1
Chromosomes coil and compact
Homologous pairs come together as pairs by synapsis
Non-sister chromatids exchange genetic makeup by Crossing over
Metaphase 1
Tetrads align at the cell equator
Anaphase 1
Homologous pairs separate and move towards the opposite poles of the cell
Telophase 1
A nuclear envelope re-forms around chromosomes in some species
Duplicated chromosomes have reached the poles
Meiosis 2
Prophase 2
Chromosomes coil and become compact (if uncoiled after telophase 1)
Nuclear envelope, if re-formed, breaks up again
Metaphase 2
Duplicated chromosomes align at the cell equator
Anaphase 2
Sister chromatids separate
Chromosomes move toward opposite poles
Telophase 2
Chromatids have reached the poles of the cells
A nuclear envelope forms around each set of chromosomes
With Cytokinesis four haploid cells are produced
Origins of genetic variation
Independent orientation at metaphase 1
Crossing over
Random fertilization
Independent orientation at metaphase 1
Each pair of chromosomes independently aligns at the cell equator
There’s an equal probability of maternal or paternal chromosome facing a given pole
The number of combinations for chromosomes packaged into gametes in 2^n where n = the haploid number of chromosomes
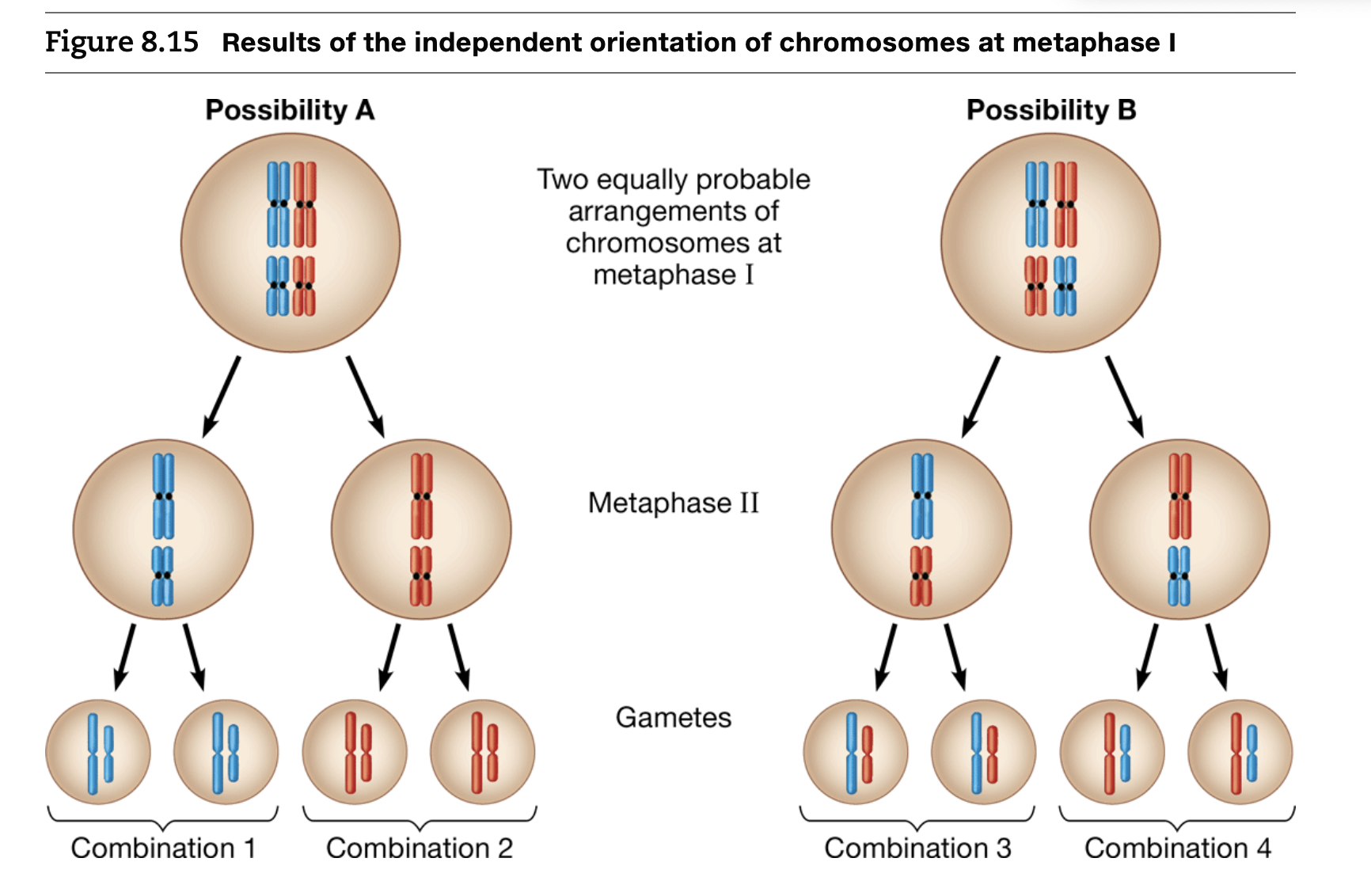
Random fertilization
The combination of each unique sperm with each unique egg increases genetic variability
Separation of homologous chromosomes during meiosis can lead to genetic differences between gametes
Homologous chromosomes may have different versions of a gene at the same locus
One version was inherited from the maternal parent and the other from paternal parent
Since homologues move to opposite poles during anaphase 1, gametes will receive either the maternal or paternal version of the gene
Genetic recombination- The production of new combinations of genes due to crossing over
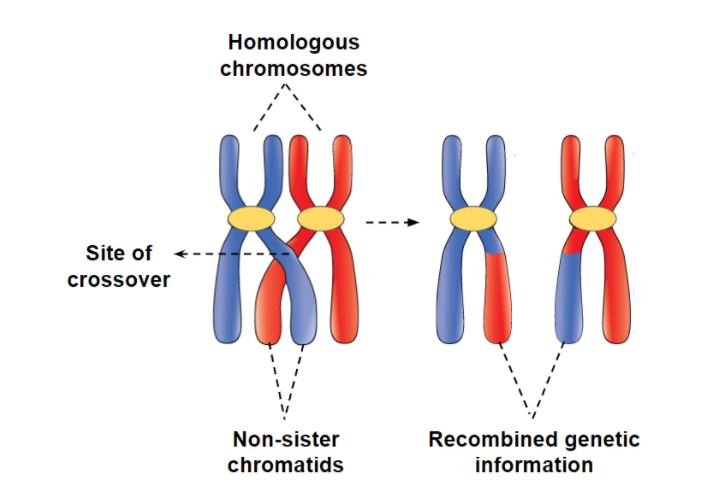
Crossing over- an exchange of corresponding segments between separate (non-sister) chromatids on homologous chromosomes
Non-sister chromatids join at the Chiasma- The site of attachment and crossing over
Corresponding amounts of genetic material are exchanged between maternal and paternal (non-sister) chromatids
Aneuploidy- Not a good set of genes 2n = 45 or 2n = 47
Karyotype- Images of a person’s chromosomes in pairs
Karyotypes are produced from dividing cells arrested at metaphase of mitosis
Karyotypes allow the observations of
homologous chromosomes
chromosome numbers
chromosome structure
Amniocentesis- Diagnostic test that shows the chromosomes of an unborn child
Trisomy 21/ Down syndrome- inheriting 3 copies of the 21st chromosome
Down syndrome is the most common human chromosome abnormality
The characteristic set of symptoms for down syndrome include:
mental retardation
short stature
circulatory defects
susceptibility to respiratory diseases, leukemia and alzheimer’s
shortened life span
characteristic facial features
The incidence of down syndrome increases with the age of the mother
Non disjunction- failure to separate during anaphase 1 or anaphase 2
Anaphase 1- all the gametes have incorrect chromosome numbers
Anaphase 2- there’s a 50/50 chance that a gamete with the right number of chromosomes will be fertilized
Fertilization after non disjunction yields zygotes with altered number of chromosomes
Abnormalities in sex chromosomes tend to be less severe because of:
the small size of the Y chromosome
the X chromosome inactivation
A single Y chromosome is enough to produce maleness even in combination with several X chromosomes
The absence of a Y chromosome yields femaleness
Errors in mitosis or meiosis may produce polyploid species with more than two chromosome sets
The formation of polyploid species are more observed in plant species compared to animals
chromosome breakage can lead to rearrangements that produces:
genetic disorders/ birth defects
if changes are in somatic cells then cancer
Rearrangements that changes the DNA sequence that changes the product includes:
Deletion- the loss of chromosome segment
Duplication- the repeat of a chromosome segment
Duplication usually of crossing over where one chromosome takes both copies of the segment inserted of exchanging segments
Inversion- the reversal of a chromosome segment
Translocation- when a segment of a chromosome separates and reattaches itself to a different chromosome
Chronic myelogenous leukemia (CML)
is one of the most common leukemias
affects cells that give rise to white blood cells (leukocytes)
results from the translocation between the 22nd and 9th chromosomes
the translocation creates a cancer causing gene called the philadelphia chromosome
Chapter 9
Pan-genesis, a concept made up by Hippocrates around 400BCE, was an early explication for inheritance that suggested that:
particles called pan-genes came from all parts of the organism to incorporated into eggs/sperm
characteristics aquifers during the parents lifetime could be transferred of the offspring
Aristotle rejected the idea and suggested that instead of particles, the potential to produce the traits was inherited
The idea that hereditary materials mix in forming offspring is called the bending hypothesis. it was:
suggested in the 19th century by a scientist studying plants
later rejected because it didn’t explain how traits that disappear in one generation can reappear in later generations
Heredity- the transmission of traits from one generation to the next
Genetics- is the scientific study of heredity
Gregor Mendel:
began the field of genetics in the 1860s
deducted the principles of genetics by breeding garden peas
relied upon the background of math, physics and chemistry
In 1866, Mendel
correctly argued that parents pass on to the offspring discrete heritable factors
stressed that the heritable factors today called genes retain their individuality generation after generation
Character- a heritable feature that varies among individuals, ex: flower color
Trait- each variant of a character, ex: purple or white flowers
True breeding varieties result when self fertilization produces offspring all identical to the parent
Hybrids are offsprings of two different varieties
The cross fertilization is a hybridization or genetic cross
O generation- true breeding parental plants
F1 generation- the hybrid offsprings of gen p
F2 generation- the cross of F1
Monohybrid cross- the cross between two individuals different in a single character
Mendel performed a monohybrid cross between with and purple flowers:
the F1 produced all purple flowers
the cross of F1 with each other made an F2 generation with ¾ purple and ¼ white flowers
The F1 generation didn’t produce any light purple flowers as predicted by the bending hypothesis
Mendel developed four theories based on his experiments:
Alleles- are alternative versions of genes that account for variations in inherited characters
For each characteristic an organism inherits two alleles one from each parent. The alleles can be the same or different
Homozygous- genotype has identical alleles
Heterozygous- genotype has two different alleles
If the alleles are heterozygous one will be dominant (determine the appearance) and one will be recessive (no noticeable effect)
phenotype- the appearance of a trait
genotype- the genetic makeup of a trait
The same phenotype may be determined by more than one genotype
The law of segregation- a sperm or egg carries only one allele for each character because the allele pairs segregate during gamete production
Mendel’s hypothesis also explains the 3:1 ratio in the F2 generation
Punnet square- shows the four possible combinations of alleles that could occur when the gamete combine
Locus(loci)- the specific location of a gene along a chromosome
Homozygous individuals have the same allele on both homologues
Heterozygous individuals have a different allele on each homologues
Dihybrid cross- a mating of parental varieties that differ in their characters
After his dihybrid cross, Mendel needed to explain why the F2 gen
had new no parental combinations of traits
and a 9:3:3:1 phenotype ratio
Mendel suggested that:
the inheritance of one character has no effect on the inheritance of another
the dihybrid cross is equivalent to two monohybrid crosses
Called this The law of independent assortment
Testcross- the mating between and individual with unknown genotype and homozygous recessive individual
A testcross can show whether the unknown genotype includes a recessive allele
Mendel used testcrosses to verify that he had true breeding genotypes
Many inherited disorders in humans are controlled by a single gene
Recessive inheritance:
two recessive alleles need to show disease
heterozygous parents are carriers of the disease causing allele
the possibility of inheritance increases with inbreeding
Dominant inheritance:
one dominant allele is needed to show disease
dominant lethal alleles are usually eliminated from the population
The most common fatal genetic disease in the US is Cystic fibrosis
The CF allele is recessive and carried by 1 in 31 Americans
Dominant human disorders include:
Achondroplasia- results in dwarfism
Huntington’s disease- caused by a late acting lethal dominant allele, degenerative disorder of the nervous system
Technology offers ways to obtain genetic information:
before conception
during pregnancy
after birth
Genetic testing can identify potential parents who are heterozygous carriers of certain diseases
Technology used before birth
Amniocentesis- extracts samples of amniotic fluid containing fetal cells and allows
karyotyping
biochemical tests for other conditions like taysachs
Chronic collusion sampling- removes a sample of chorionic villus tissue from the placenta and allows karyotyping and biochemical tests
Chronic collusion sampling takes shorter to produce results
Fetal imaging allows the physical to examine a fetus directly for anatomical deformities
The most common form of fetal imaging is ultrasound imaging
Newborn screening can detect diseases that can be prevented by special care and precautions
Ethical considerations of the technologies
confidentiality and potential use of results of genetic testing
time and financial costs
determining what, if anything, should be done as a result of testing
Variations on Mendel’s Laws
Complete dominance- when the offspring always looked like on of the parental varieties
Incomplete dominance- when the phenotype of the offspring falls between the two parental varieties:
Neither allele is dominant over the other
Both alleles are expressed and results in a 3rd intermediate phenotype
A heterozygote expressing an intermediate phenotype= incomplete dominance occurred
Incomplete dominance does not support the bending hypothesis because the original parental phenotypes reappear in the F2 generation
ex Incomplete dominance in humans:
hypercholesterolemia
extremely high levels of cholesterol occurs in blood
heterozygotes have intermediately high cholesterol levels
Although an individual can carry at most 2 different alleles for a particular gene, more that two alleles can exist in the wider population
The human ABO blood group phenotype has three alleles for a single gene
The four blood groups A,B,AB,O are phenotypes resulting from the two alleles
A and B are both expressed in heterozygous individuals in Codominance
In Codominance:
neither allele is dominant over the other
both alleles are expressed in distinct phenotypes
EX: Type AB blood
difference between codominance and incomplete dominance: codominance doesn’t have intermediate phenotypes
Mendel knew that the rules of mathematical probability affected:
The segregation of allele pairs during gamete formation
The re-forming of pairs at fertilization
The probability scale ranges from 0 to 1
certainly has the probability of 1
certainly not has the probability of 0
Probability of a specific event- the number of ways that event can occur out of the total possible outcomes
When determining the probability of individual events use the rule of multiplication where the product is the probabilities for each event
The probability that an event can occur in two or more alternative ways is the sum of the separate probabilities called rule of addition
Wild-type traits- traits that aren’t necessarily specified by dominant alleles
Some dominant and recessive traits
Freckles are dominant over no freckles
Widows peak is dominant over straight hairline
Free earlobe is dominant over attached earlobe
The inheritance of human traits follows Mendel’s laws
A Pedigree is used to:
Show the inheritance of a trait in a family through multiple generations
demonstrates dominant or recessive inheritance
can be used to deduce genotypes of family members
can be used on both autosomal and sex linked traits
Even though a person can carry at most 2 alleles of one gene more that 2 alleles can exist in the wider population
The human blood group phenotypes have 3 alleles for a single gene
The phenotypes: A, B, AB, O
A and B are codominant
In Codominance:
One allele isn’t dominant over the other
both phenotypes are expressed in distinct phenotypes
Pleiotropy- when one gene influences many characteristics
ex: sickle cell - affects:
affects the shape of red blood cells
causes anemia and organ damage
makes a person resistant to malaria
sickle cell is co-dominant to normal cell
Polygenic inheritance- when a single phenotypic character results from the combined effects of two or more genes
ex: skin color, height, weight, hair color, eye color
Epistasis- When one unrelated gene can affect the expression of all of the other genes for that trait
Ex: albino genes, gene for baldness
Many characters are products of heredity and environment
ex:
skin color is affected by sunlight exposure
susceptibility to cancer has both hereditary and environmental factors
identical twins show some differences
Only genetic influences are inherited
The Chromosome theory of inheritance states that:
genes take up specific loci on chromosomes
Chromosomes segregate and independently assort during meiosis
Mendel’s laws correlate with chromosome separation in meiosis
the law of segregation depends on homologous chromosomes in anaphase 1
the law of independent assortment depends on alternative orientations of chromosomes in metaphase 1
Bateson and Punnett studied plants that didn’t show a 9:3:3:1 ratio in the F2 generation and accidentally discovered linked genes
linked genes- genes on the same chromosome
They tend to be inherited together
The closer in proximity the genes are the greater the likelihood that they’re inherited together
Linked genes don’t follow Mendel’s laws of independent assortment
Even with crossing over, some traits are almost always inherited together
Crossing over between homologous chromosomes produces new combinations of alleles in gametes
Linked genes that are separated by crossing over form recombinant gametes
The percentage of recombinant is the recombination frequency
recombinants- offsprings whose genotypes don’t match the parents
Recombination frequency (%) = recombinants / total number of offspring
Geneticists use crossover data to map genes
The more genes are farther away from each other the higher the rate of crossover can occur
Recombination frequencies can be used to map the relative location of genes on chromosomes
ex: if 2 genes that cross over 20% of the time are 20 map units apart
Many animals have a pair of sex chromosomes
In mammals:
males have and XY chromosomes
females have and XX chromosomes
The Y chromosome contains SRY genes that code for testosterone, all the male reproductive systems and sex characteristics like deep voice, facial hair, larger muscles
The absence of Y chromosomes allows the female reproductive system to develop
The X chromosome is much larger and carries additional traits that are not connected to biological sex
different animals have different ways of determining biological sex
ex:
Grasshoppers, roaches and some other insects have an X-O system where O stands for the absence of a sex chromosome
Females are XX
Males are XY
In some fishes, butterflies and birds the sex chromosomes are Z and W
Females are ZW
Males are ZZ
Some organisms lack sex chromosomes all together
ex:
Females are diploid
Males are haploid
In some animals environmental temperature determines biological sex
ex:
In some species of reptiles the biological gender is determined by the temperature at which the eggs are incubated at during a period of development
Global climate change heavily impacts the sex ratio of the species
Sex linked genes- genes located on either of the sex chromosomes
Sex linked traits- traits inherited on the X chromosome
Males and females will express sex linked traits differently
Males are more likely to express a sex linked disorder compared to females
Males can’t be carriers of sex linked disorders, they either have it or not
The X chromosomes carries many genes that are not related to biological sex
Most sex linked human disorders are:
caused by recessive alleles
mostly seen in males
A male will have the disorder if he receives a recessive allele from his mother
A female will have the disorder if she receives a recessive alleles from both parents
Examples of recessive sex linked disorders
hemophilia- lack of proteins needed for blood clotting
red-green color blindness- a malfunction of light sensitive cells in the eyes
Duchenne muscular dystrophy- the progressive weakening of the muscle and loss of coordination
The Y chromosome provides clues about human male evolution because:
Y chromosomes are passed intact from father to son
mutations in the Y chromosome can reveal data about recent shared ancestry
Chapter 10
10.1
Until the 1940s scientists believed that protein served as genetic material was stronger than DNA
Proteins were made from 20 different amino acids
DNA was just made from four kinds of nucleotide
Studies of bacteria and viruses
helped in molecular biology- the study of heredity at a molecular level
revealed the role of DNA in heredity
In 1928, Frederick Griffith discovered that a “transforming factor” could be transferred into a bacteria cell
He discovered that:
when a heat killed pathogenic(disease causing) bacteria is exposed to a harmless bacteria, some harmless bacteria would be converted to disease-causing bacteria
the disease causing characteristic was inherited by the descendants of the transformed cells
In 1952, Alfred Hershey and Martha Chase used bacteriophages to show that DNA is the genetic material of T2(a virus that infects that bacterium E. coli)
Bacteriophages (phages)- are viruses that infect bacteria cells
Phages were labeled with radioactive sulfur to detect proteins or radioactive phosphorus to detect DNA
Bacteria were infected with either type of phages to determine which substance was injected into the cell and which remained outside the cell
The sulfur labeled protein stayed with the phages outside the bacteria cell and the phosphorus labeled DNA was detected inside cells
Cells with phosphorus labeled DNA produced new bacteriophages with radioactivity in DNA but not in protein
10.2
DNA and RNA are nucleic acids and are polymers of nucleotides
DNA polynucleotide- A nucleotide chain. One of the two strands of DNA
A Nucleotide is made of
nitrogen base
five carbon sugar called deoxyribose
phosphate group
Nucleotides are joined to one another by a Sugar phosphate backbone
There are four nitrogen bases that can make up a DNA nucleotide
Adenine (A)
Cytosine (C)
Thymine (T)
Guanine (G)
RNA is different from DNA in that it has:
the sugar ribose instead of deoxyribose
RNA has a nitrogen base uracil (U) instead of thymine
10.3
After the Hershey-Chase experiment there was a race on to
describe the structure of DNA
explain how the structure on properties of DNA can account for its role in heredity
In 1953, James D. Watson and Francis Crick deduced the secondary structure of DNA using
X-ray crystallography data of DNA from the work of Rosalind Franklin and Maurice Wilkins
Chargaff’s observation that in DNA
the amount of adenine was equal to the amount of thymine
the amount of guanine was equal to the amount of cytosine
Watson and Crick reported that DNA consisted of two polynucleotide strands wrapped into a double helix
the sugar-phosphate backbone is on the outside
the nitrogenous bases are perpendicular to the backbone in the interior
specific pairs of bases give the helix a uniform shape
A pairs with T forming two hydrogen bonds
G pairs with C forming three hydrogen bonds
In 1962, the Nobel prize was awarded to
James D. Watson, Francis Crick and Maurice Wilkins
Rosalind Franklin probably would’ve received the prize as well but she died in 1958
The Watson-Crick model gave new meaning to the words genes and chromosomes. The genetic information in a chromosome is encoded in the nucleotide sequence of DNA
10.4
In their description of DNA, Watson and Crick noted that the structure of DNA suggests a possible copying mechanism
DNA replication is Semiconservative
the two strands separate
each strand is used as a template to produce a complementary strand using the base pairing rule
each new DNA helix has one old and one new strand
10.5
DNA replication begins at the origin of replication where:
DNA unwinds at the origin to produce a “bubble”
replication proceeds in both directions from the origin
replication ends when products from the bubbles merge with each other
DNA replication occurs in the 5’ to 3’ direction adding new nucleotides to the 3’ end
Replication is continuous (leading) on the 3’ to 5’ template
Replication is discontinuous (lagging) on the 5’ to 3’ template, forming short segments
The key enzymes involved in DNA replication
Hilacase- separates the parent DNA strand
Primase- initializes the process of replication (made of RNA)
DNA polymerase- adds new nucleotides to the growing chain based on the parent template, proofreads and corrects incorrect base pairings
Ligase- connects the Okazaki fragments (lagging strands)
DNA polymerase and Ligase also repair DNA damaged by harmful radiation and toxic chemicals
DNA replication ensures that all the somatic cells in a multicellular organism carry the same genetic information
10.6
DNA specifies traits by dictating protein synthesis
The molecular chain of command is from
DNA in the nucleus to RNA and
RNA in the cytoplasm to protein
Transcription- the synthesis of RNA under the directions of DNA
Translation- the synthesis of protein under the directions of RNA
The DNA genotype os expressed as proteins which provide the molecular basis for phenotypic traits
The connections between genes and proteins
The initial one gene - one enzyme hypothesis was based on studies of inherited metabolic diseases
One gene - one enzyme hypothesis was expanded to include all proteins
Recently the one gene - one polypeptide hypothesis recognizes that some proteins are composed of multiple polypeptides, also recognizes that genes code for many proteins not just enzymes
10.7
Genetic information written in codons is translates into amino acid sequences
The sequence of nucleotides in DNA provides a code for constructing a protein
protein construction needs a conversion of a nucleotide sequence to an amino acid sequence
The flow of information from gene to protein is based in a triplet code called codons
Translation involves switching from the nucleotide language to amino acid language
Each amino acid is specified by a codon
64 codons are possible
some amino acids have more than one possible codon
10.8
The genetic code dictates how codons are translated into amino acids
Characteristics of the genetic code
Three nucleotides specify one amino acid
61 codons correspond to amino acids
AUG codes for Methionine and signals the start of transcription, codes for an amino acid
3 stop codons signal the end of translation, they don’t code for an amino acid
The genetic code is
Redundant- more than one codon for some amino acids
Unambiguous- any codon for one amino acid doesn’t code for any other amino acid
Nearly universal- the genetic code is shared by most organisms
Without punctuation- codons are adjacent to each other with no gaps in between
10.9
Transcription produces genetic messages in the form of RNA
RNA synthesis resembles the synthesis of DNA during DNA replication
RNA nucleotides are linked by the transcription enzyme RNA polymerase
Specific sequences of nucleotides along the DNA mark where transcription begins and ends
The start transcribing signal is a nucleotide sequence called a prompter
There are 3 phases in RNA synthesis
Transcription begins with initiation as the RNA polymerase attaches to the prompter
During the second phase elongation the RNA grows longer
As the RNA peels away, the DNA strands rejoin
In the third phase termination the RNA polymerase reaches a sequence of bases in the DNA template called a terminator which signals the end of the gene
The polymerase molecule detaches from the RNA molecule and the gene
RNA is synthesized in a 5’ to 3’ direction
10.10
Eukaryotic RNA is processed before leaving the nucleus as mRNA
mRNA
encodes for amino acid sequences
conveys genetic messages from DNA to the translation machinery of the cell which in:
prokaryotes- occurs in the same place that mRNA is made
transcription and translation happens at the same time
leads to more errors
eukaryotes- mRNA must exit the nucleus via nuclear pores to enter the cytoplasm
more sequenced and accurate
Eukaryotic mRNA has
introns- interrupting sequences that separate exons
Non coding segments
exons- the coding regions
Eukaryotic mRNA undergoes RNA processing before leaving the nucleus
RNA processing/ RNA splicing
removes introns and joins exons to produces a continuous coding sequence
A cap and tail of extra nucleotides are added to the ends of the mRNA to:
facilitate the export of the mRNA from the nucleus
protect the mRNA from attack by cellular enzymes
help ribosomes bind to the mRNA
10.11
transfer RNA molecules serve as interpreters during translation
tRNA- molecules function as a language interpreter
converting the genetic messages of mRNA
into the language of proteins
tRNA molecules perform this interpreter task by
picking up the appropriate amino acid
using a special triplet of bases called anticodon, to recognize the appropriate codons in the mRNA
tRNA has 2 binding sites
anticodon- binds with the mRNA codon
amino acid- the amino acid product exits
10.12
Ribosomes build polypeptides
Translation occurs on the surface of the ribosome:
Coordinate the functioning mRNA and tRNA and ultimately the synthesis of polypeptides
have two subunits: small and large
each subunit is made of rRNA and proteins
ribosomal subunits come together during translation
ribosomes have binding sites for mRNA and tRNAs
10.13
Translation can be divided into the same three phases as transcription:
initiation
brings together
mRNA
a tRNA bearing the first amino acid
the two subunits of a ribosome
establishes where translation will begin
occurs in two steps:
mRNA molecule binds to a small ribosomal subunit and the first tRNA binds to the mRNA at the start codon
the start codon reads AUG and codes for methionine
the first tRNA has the anticodon UAC
A large ribosomal subunit joins the the small subunit allowing the ribosome to function
the first tRNA occupies the p site, which will hold the growing peptide chain
the a site is available to receive the next tRNA
Elongation
adds amino acids one by one to the polypeptide chain
Each cycle of elongation has three steps
codon recognition- the anticodon of an incoming tRNA molecule carrying its amino acid pairs with mRNA codon in the A site of the ribosome
peptide bond formation- the new amino acid is joined to the clan
translocation- tRNA is released from the P site and the ribosome moves tRNA from the A site into the P site
Elongation continues until the termination stage of translation when,
the ribosome reaches a stop codon
the completed polypeptide is freed from the last tRNA
the ribosome splits back into its separate subunits
Termination
ends translation
10.15
4 major processes
replication- DNA to DNA
transcription- DNA to RNA transcript
RNA processing- produces mRNA
translation- mRNA to protein
10.16
Mutations can change the meaning of genes
A mutation is any change in the nucleotide sequence of DNA
mutations can involve
large chromosomal regions or
just a single nucleotide pair
Mutations can be divided into two general categories
Substitution
involves the replacement of one nucleotide with another
may not effect at all- silent mutation
changes the amino acid coding which produces a different amino acid- missense mutation
lead to a base substitution that produces an improved protein that enhances the success of the mutant organism and its descendants
change an amino acid into a stop codon, produces a nonsense mutation
Deletions/ insertions
may alter the reading frame (triplet grouping) of the mRNA so that nucleotides are grouped into different codons
lead to significant changes in amino acid sequence downstream of the mutation
produce a nonfunctional polypeptide
Mutagenesis- the production of mutations
Mutations can be caused by
spontaneous errors that occur during DNA replication or recombination
mutagens, includes
high energy radiation such as X rays and ultraviolet light
chemicals
Chapter 13
Evolution- Change in allele frequency over time
driven by natural selection
only populations/ species evolve
Allele- Version of a trait
Natural selection- The mechanism that allows evolution to take place
Easier to occur with sexually reproducing organisms
Requirements for natural selection include:
Variation must exist
Limited resources
Competition
Some organisms with genes that are better adapted to survive (determined by DNA)
Survival→Reproduction→Pass on trait
Organisms that don’t have the beneficial traits die off and decrease their allele frequency and over time the population changes
Fossils- the imprints or remains of organisms that lived in the past
Jean Baptiste Lamarck’s proposal
organisms evolve by the use and disuse of body parts
the acquired characteristics are passes on to their offspring
Lyell’s Principles of Geology
natural forces gradually changed earth
natural forces are still operating today
During his voyage, Darwin realized that
The earth is very old
Over time, present day species have arisen from ancestral species by natural processes
Darwin published On the Origin of Species by Means of Natural Selection
Main ideas of the book:
it presents a logical explanation of descent with modification and evolution by natural selection
organisms accumulate various adaptations that fit their environments
exploring adaptations of organisms to their environment
discussed examples of natural selection
recognized the connection between natural selection and the capacity to over reproduce
Key points of evolution by natural selection
Individuals don’t evolve, they either survive or die
Natural selection can only amplify or diminish only heritable traits not acquired characteristics
Evolution doesn’t lead to perfection, favorable traits as environments change
Main points about natural selection
Natural selection is more of an editing process that a creative mechanism
Natural selection is contingent on time and place
Fossil record- the sequence in which fossils appear within strata(layers of sedimentary rocks)
Paleontologist- scientists who study fossils
Scientist have found that prokaryotic cells→eukaryotic cells→multicellular eukaryotic cells
Biogeography- the geographic distribution of species
Comparative embryology- the comparison of early stages of development among different organisms. It reveals homologies not visible in adult organisms
Vestigial structures- remnants of features that served important functions in an organism’s ancestors
Molecular biology- used to reveal evolutionary relationships by comparing DNA and amino acid sequences between different organisms
Darwin was the first to represent the history of life as a tree
Homologous structures can be used to determine the branching sequences of an evolutionary tree. This includes:
anatomical structure
molecular structure
Population- a group of individuals of the same species and living in the same place at the same time
Populations maybe isolated from one another with interbreeding
Gene pool- the total collection of genes in a population at any one time
Microevolution- a change in the relative frequencies of alleles in a gene pool over time
Population genetics- studies how populations change genetically over time
Modern synthesis- connects Darwin’s theory with population genetics
Organisms typically show individual variation
The Origin of Species couldn’t explain:
the cause of variation among individuals
how variations were passed down from parents to offspring
Mutations:
changes in the nucleotide sequence of DNA
the ultimate source of new alleles
Chromosomal duplication is an important source of genetic variation
Sexual reproduction shuffles alleles to reproduce new combinations in three ways:
Independent assortment
Crossing over
Random fertilization
Hardy-Weinberg principle- within a sexually reproducing diploid population allele and genotype frequencies will remain in equilibrium unless outside forces act to change those frequencies
The conditions of Hardy-Weinberg
A very large population
No gene flow between populations
No mutations
Random mating
No natural selection
Genotype frequencies- p² + 2pq + q² = 1
Alleles- p + q = 1
The Hardy-Weinberg equation is useful in public health science
The three main causes of evolutionary change aka microevolution
Natural selection
Genetic drift
Gene flow
Genetic drift:
a change in the gene pool of a population due to change
in a small population it may lead to the loss of genetic diversity
bottleneck effect: leads to a loss of genetic diversity when a population is greatly reduced
founder effect: when a few individuals colonize a new habitat
Relative fitness: it makes to the gene pool of the next generation relative to the contribution of other individuals
Natural selection can affect the distribution of phenotypes in a population
Stabilizing selection- favors intermediate phenotype, against extreme phenotypes
Directional selection- acts against individuals to one end of the phenotypic extremes
Disruptive selection- favors individuals at both extremes, against intermediate phenotypes
Sexual selection- a form of natural selection where individuals with certain characteristics are more likely to obtain mates
Sexual dimorphism- the different appearances if male and female species
Intersexual selection(b/n same sex)- competition for mates, usually by males
Intersexual selection(b/n sexes) aka mate choice- when individuals of one sex(usually females) are choosy in piking their mates and often select flashy or colorful mates
What prevents natural selection from eliminating unfavorable genotypes
Natural selection attacks phenotypes not genotypes so heterozygous individuals survive
Balancing selection- maintains stable frequencies of two or more phenotypes in a population
Heterozygous advantage
Frequency-dependent selection- a type of selection that maintains two different phenotypes in a population
Chapter 37
Biological community:
an assembly of all the population of organisms living close enough together for potential interaction
described by its species composition
Interspecific interactions:
relationship with individuals of other species in the community
greatly affect population structure and dynamics
can be categorized according to their effect on the interacting populations
Interspecific competition:
occurs when populations of two different species compete for the same limited resource
Mutualism- both populations benefit
Predation- one organism kills and eats the other organism
Herbivory- an animal consumes plant parts or algae
Parasitism- the host plants or animals are victimized by parasites or pathogens
Ecological niche- the sum of an organisms’s use of the biotic and abiotic resources in its environment
Interspecific competition occurs when the niches of two populations overlap
Competition lowers the carrying capacity of competing population b/c the resources used by one population aren’t available to the other population
Predication leads to diverse adaptations in prey species like:
camouflage
mechanical defenses
chemical defenses
Herbivores and plants undergo coevolution
Coevolution- a series of reciprocal evolutionary adaptations in two species in which change in one species acts as new selective force on another
Herbivory leads to diverse adaptations in plants
Parasites and pathogens can affect Community composition
Parasite- lives in or in a host from which it obtains nourishment
Pathogens- disease causing microscopic parasite that include
bacteria
viruses
fungi
protists
Non-native pathogens can have rapid and dramatic impacts
Non-native pathogens can cause a decline of the ecosystem
Trophic structure- pattern of feeding relationships within a community
Food chain- the sequence of food transfer up the trophic levels
Producers- autotrophs that support all the other trophic levels
Consumers- heterotrophs
Primary consumers
Tertiary consumers
Quaternary consumers
Detritiviores- get the energy from detritus(the dead material produced at all trophic levels)
Decomposers- have enzymes that digest organic materials and convert them into inorganic forms in the process of decomposition. mainly prokaryotes and fungi
10% rule- only 10% of the energy from one tier gets transferred from one level to the next
Food web- a network of interconnecting food chains
consumers may eat more than one type of producer and several species of consumers may feed on the same species of producer
Species diversity: defined in two components
species richness, the number of species in a community
relative abundance , the proportional representation of a species in a community
Keystone species- a species whose impact on its community is larger than its biomass or abundance indicates and occupies a niche that holds the rest of its community in place
keystone species have a disproportionate impact on diversity
Disturbances: events that damage biological communities. the frequencies and severity may vary from community to community
Communities change drastically following a severe disturbance that:
strips away vegetation
removes significant amount of soil
Ecological succession- results from colonization by variety of species whisch are replaced by a succession of other species
Primary succession- begins in a lifeless area with no soil
Secondary succession- occurs when a disturbance destroys an existing community by leaves the soil intact
Invasive species- organisms that have bben introduced into non-native habitats by human actions
Invaisive species establish themselves at the expense of native communities
The absence of natural enemies → rapid growth of invaisive species
Ecosystem- consists of all the organisms in a community and the abiotic envrionment with the organisms interact
Energy flow- moves through the components of an ecosystem
Chemical cycling- the transfer of materials within the ecosystem
Terrarium- represents the components of an ecosystem and illustrates the fundamentals of energy flow
Primary production:
carried out by producers
the amount of solar energy converted to chemical energy by an ecosystem’s producers for a given area
produces biomass-the amount of living organic material in an ecosystem
Ecosystems vary in their primary production and contribution to the total production of the biosphere
Energy supply limits the length of food chains
Chemicals are cycled between organic matter and abiotic reservoirs
Ecosystems get their energy from:
the sun
the earth’s core
Biogeochemical cycles: include
biotic components
abiotic components
abiotic reservoirs- where a chemical accumulates outside of living organisms
Biogeochemical cycles can be local or global
Carbon cycle
Carbon is found in
the atmosphere
fossil fuels
dissolved in carbon compounds in the ocean
The return of CO2 to the atmosphere by respiration closely balances its removal by photosynthesis
The carbon cycle can be affected by things like:
burning wood
burning fossil fuels
Steps of the Carbon cycle
Carbon enters the atmosphere
Plants absorb CO2
Carbon enters the food chain
Carbon reenters the atmosphere
Sources of carbon to the atmosphere:
burning
decomposition
respiration
Phosphorus cycle
The phosphorus cycle doesn’t have an atmospheric component
Rocks are the only source of phosphorus for ecosystems
Plants absorb phosphorus ions in the soil and build them into organic compounds
Phosphorus are returned ti the soil by decomposers
Phosphorus levels in aquatic ecosystems are typically low enough to be a limiting factor
Nirtogen cycle
nitrogen has 2 abiotic reservoirs
the atmosphere
soil
Nitrogen fixation:
converts N2 compounds of nitrogen that can be used by plants
is carried out by some bacteria
Decomposers- use their enzymes to change the dead living organims into Ammonium by using the process of decomposition
Denitrifiers- converts nitrate and nitrite in the soil into nitrogen gas that enter the atmosphere by using the process of denitrification
Nitrifying bacteria- converts the ammonia into nitrate by using the process of nitification
In aquatic ecosystems, primary production is limited by low levels of:
Phosphorus
Nitrogen
A rapid inflow of nutrients degrades aquatic ecosystems
Over time standing water ecosystems
gradually accumulate nutrients from the decomposition of organic matter
primary production increases in a process known as Eutrophication
Eutrophication depletes oxygen levels and decreases species diversity
Phosphate pollution leading to eutrophication comes from:
fertilizers
pesticides
sewage treatment facilities
runoff of animal waste
feedlots
Although we depend on agricultural ecosystems we also get resources from natural ecosystems
Examples of natural ecosystems:
supply of freshwater and some foods
recycling nutrients
decomposition of waste
regulation of climate and air quality
A rapid increase of food production comes in the expense of natural ecosystems and the resources they produce
Human activities also threaten many ecosystems and their products
 Knowt
Knowt
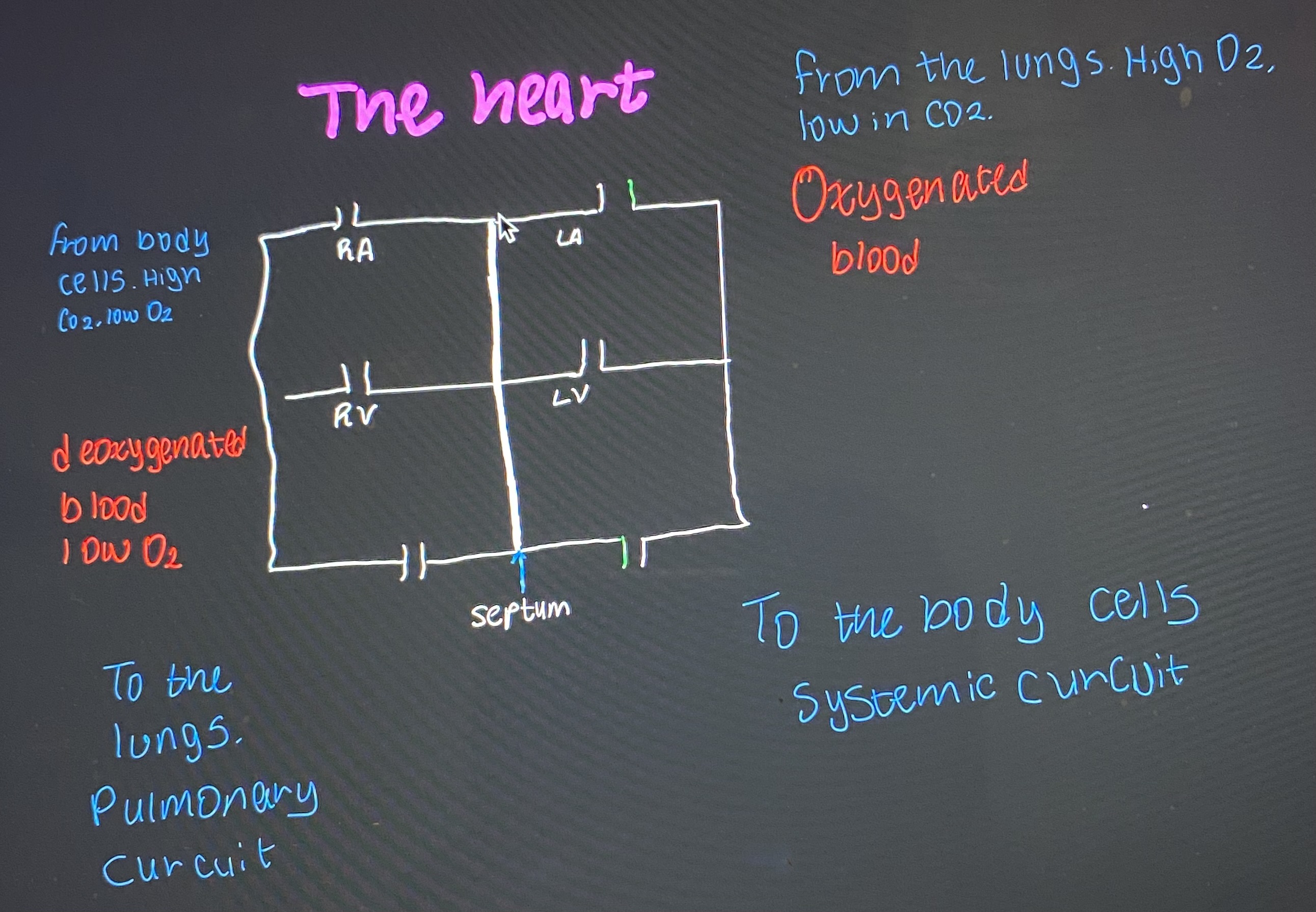 The hear has four chambers
The hear has four chambers