CHEM122 Chemical Equilibrium
Chemical Equilibria and Reactions
Overview of Chemical Reactions
Recall, chemical reactions and stoichiometry: precipitation reactions, acid-base reactions, oxidation-reduction reactions.
All reactions we considered were assumed to be unidirectional.
Equilibria and Reaction Rates
All reactions are, in theory, equilibria!
Some reactions are just more 'equal' than others.
Introduction to Chemical Equilibria
Overview of Equilibria
In this section we will focus on systems which have measurable equilibria.
Chemical equilibria are dynamic processes and reactions are always occurring.
Equilibrium Dynamics
At equilibrium, the concentrations of both A and B are invariant with time.
Properties of Chemical Equilibria
Competing Reactions
Consider the following competing reactions:
Forward reaction: H2O + CO → H2 + CO2
Reverse reaction: H2 + CO2 → H2O + CO
Rate of forward reaction = kf[H2O][CO]
Rate of reverse reaction = kr[H2][CO2]
At equilibrium, forward and reverse rates are identical.
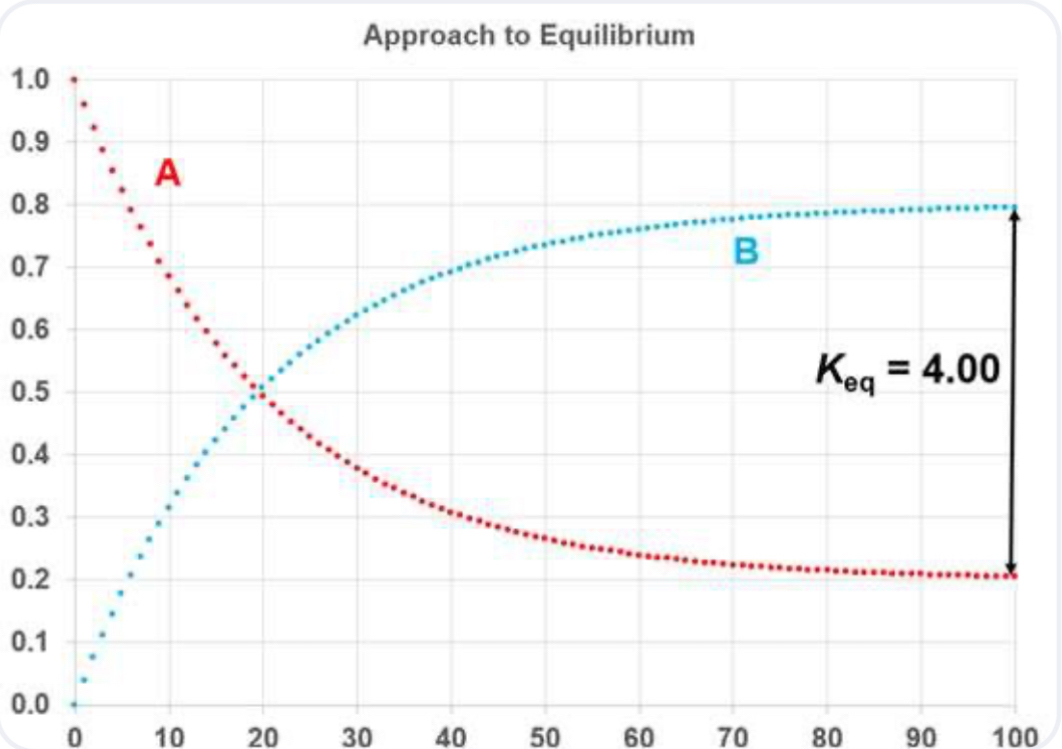
Equilibrium Constant
At equilibrium, forward rate = backward rate: kf[H2O][CO] = kr[H2][CO2].
Equilibrium constant K (capital K - state function) is the ratio of the forward to backward rate constants: K = kf/kr.
Properties of Equilibrium Constants
General Properties
If the reaction is written in reverse, the new equilibrium constant is simply the reciprocal of the first constant: K' = 1/K.
If the reaction equation is multiplied by some factor (e.g., n), K is raised to the power of that factor: K' = K^n.
The equilibrium of a stepwise reaction is the product of the equilibrium constants of the individual reaction steps.
Thermodynamic Nature
Equilibrium constants are thermodynamic quantities; they tell us the equilibrium position, but not how fast we reach this position.
Equilibrium Constants in Gaseous Reactions
Pressure and Concentration
For reactions involving gases, the equilibrium constant can be expressed using pressures: K = PNH3/(PH2)^3(PN2).
Conversion of Kc to Kp and vice versa is necessary.
General Case for Gases
Kc = Kp(RT)^Δn, where Δn = moles products – moles of reactants.
Industrial Applications of Equilibria
Synthesis of Ammonia
The industrial synthesis of ammonia from nitrogen and hydrogen is an important reaction (industrial catalyst is iron based).
About 160,000,000 tons is produced per year; ammonia is used to make numerous chemicals.
Reaction Conditions
The process only proceeds at high temperatures used for ammonia synthesis, around 400 ºC.
Reaction Quotient and Equilibrium
Reaction Quotient (Q)
A useful indicator for how close to equilibrium is the reaction: reaction quotient (Q).
The magnitude of K indicates the extent of reaction: large K → mostly products; small K → mostly reactants.
Outcomes of Q
Three possible outcomes:
Q = K: reaction is at equilibrium.
Q > K: reaction shifts to the left, concentration of products decreases.
Q < K: reaction shifts to the right, concentration of products increases.
Le Chatelier's Principle
Definition and Importance
Le Chatelier's principle is an important concept in equilibration.
"If a stress is imposed on a chemical system at equilibrium, the equilibrium position will shift in a direction which reduces the imposed stress."
What does this mean? Stress ≡ a change in concentration, temperature or pressure.
Effects of Changing Concentrations
Imposed stress – change in concentration.
At equilibrium: [N2] = 0.399 M; [H2] = 1.197 M; and [NH3] = 0.202 M.
Now add 1.000 mol/L N2.
Q < K: the system will shift to the right to obtain a new equilibrium position.
Summary:
If a reactant or product is added to a system at equilibrium, the system will shift away from the added component.
If a reactant or product is removed, the system will shift toward the removed component.
Addition of nitrogen or hydrogen will result in the production of more ammonia.
Effects of Changing Reaction Pressure
There are 3 ways to change the pressure:
Add or remove a gaseous reactant or product at constant volume: equivalent to a change in concentration; result: the equilibrium shifts away from added species.
Add an inert gas at constant volume: the addition of an inert gas increases the total pressure but has no effect on the concentrations or partial pressures of the reactants or products (assuming ideal behaviour); the system remains at the original equilibrium position.
Change the volume of the container: changes in the volume of the container affect the partial pressures or concentrations of the reactants and products and hence shift the equilibrium position. If the volume increases the pressure decreases and the system responds by shifting the equilibrium to increase the pressure (i.e., increase the number of gas phase molecules).
Lower the volume of the container: result: a decrease in volume increases the pressure and the system responds by shifting the equilibrium to relieve the pressure (i.e., decreases the number of gas phase molecules).
Effects of Changing Reaction Temperature
Chemical reactions are either exothermic (give out energy i.e., heat, ΔH is negative) or endothermic (absorb energy, ΔH is positive).
Reactants → products + heat energy (exothermic).
Reactants + heat energy → products (endothermic).
Consider energy as a reactant or product; the equilibrium will shift to oppose the change in temperature.
Summary:
A temperature increase forces an exothermic reaction to the left and an endothermic reaction to the right.
A temperature decrease pushes an exothermic reaction to the right and an endothermic reaction to the left.
An increase in temperature forces the equilibria in the endothermic direction; will shift to oppose the change in temperature.
Catalysis and Equilibrium Position
A catalyst speeds up both forward and reverse reactions.
The equilibrium is achieved more rapidly, but its position is unaffected by the catalyst.
Summary:
Changing the pressure or concentration will shift the equilibrium composition but does not affect the equilibrium constant.
Changing the temperature changes the value of the equilibrium constant.
Equilibrium Calculations
Setting Up Calculations
Question: F2(g) + H2(g) → 2HF(g)
At equilibrium, the concentrations are:
[HF] = 0.37 mol/L
[H2] = 1.72 mol/L
[F2] = 1.72 mol/L
If all the hydrogen fluoride is removed from the container, what will be the new equilibrium compositions in mol/L?
Equilibrium Constant Calculations
Calculating the Equilibrium Constant
First, calculate the equilibrium constant
𝐾= HଶFଶ / HF ଶ
calculate K
𝐾= HଶFଶ / HF ଶ = 1.72 × 1.72 / 0.37 ଶ = 21.6
Setting Up ICE Tables
Now set up the ICE tables for the new equilibrium concentrations
H2, F2, HF
ICE TABLE
1.72, 1.72, 0 (concinitial)
−x, −x, +2x (concchange)
1.72−x, 1.72−x, 2x (concequilibrium)
Solving for x
Calculate x from the ICE table
𝐾= HଶFଶ / HF ଶ = 1.72 −x ଶ / 4xଶ = 21.6
2.96 −3.44x + xଶ= 21.6 × 4xଶ
85.4𝑥ଶ+ 3.44𝑥−2.96 = 0
Now solve the quadratic equation
𝑥= −3.44 ± √(3.44 ଶ−4 × 85.4 × −2.96)
𝑥= −𝑏± √(𝑏ଶ−4𝑎𝑐) / 2 × 85.4
𝑥= −3.44 ± 31.98 / 170.8 = 0.17 (other root is negative, impossible)
Checking the Answer
Calculate concentrations
H2, F2, HF
ICE TABLE
1.72, 1.72, 0 (concinitial)
−0.17, −0.17, +2 × 0.17 (concchange)
1.55, 1.55, 0.34 (concequilibrium)
Now check your answer
𝐾= HଶFଶ / HF ଶ = 1.55 × 1.55 / 0.34ଶ = 20.8
Note, answer is within 5% of K, therefore rounding is fine