Randomisation Blinding Sample Size Calculations flashcards
Topic 3: Randomisation, Blinding and Sample Size Calculations
Instructor: Lizzie Chappell
Date: 4th March 2025
Fundamentals of Design for Major Randomized Controlled Trials
Choice of Patients and Centers
Set precise eligibility criteria:
Not too specific nor too broad.
Prefer large-scale, multicenter trials to ensure geographic representation.
Choice of Treatments
Specify precise treatment regimens.
Include placebo/sham control group or active comparator.
Consider a 3-arm trial for comprehensive evaluation.
Choice of Outcomes
Define the primary efficacy endpoint clearly.
List secondary endpoints.
Incorporate safety concerns into the overall outcome priorities.
Randomization
Allocation concealment is crucial to avoid bias.
Choose a statistical method for randomization.
Stratification can help ensure balance in groups but may not be key in large trials.
Consider unequal randomization favoring new treatment in certain situations.
Use of Blinding
Aim for double-blind trials when feasible:
If not, blinded evaluation required.
Especially important for subjective endpoints.
Choice of Trial Size
Use power calculations to determine required trial size.
Choose a realistic anticipated effect size.
Compromise may be needed for practical target size.
Objectives of Randomisation
Eliminate bias in treatment group allocation.
Ensure treatment groups do not differ systematically.
Balance known and unknown prognostic factors.
Aim is to allocate treatments independently of patient characteristics.
Types of Randomisation
Simple Randomisation
Randomise patients using simple methods:
E.g., Coin toss or dice rolls.
Advantages:
Quick, no special technology required.
Disadvantages:
Potential for unbalanced groups, losing statistical power.
Random Permuted Blocks
Construct a randomization list using blocks to ensure balance:
Example block sequences (length of 4): AABB, ABAB.
Maintain small differences in group sizes.
Stratification
Balances treatment groups concerning specific patient characteristics:
Common factors: center, disease stage, age, sex, disease markers.
Create separate randomisation lists for each stratum.
Minimisation
Addresses balancing multiple stratification factors:
Avoids predictability while maintaining random allocation.
Controversial due to potential predictability in allocation.
Sample Size Calculations
Importance of Sample Size
Sample Size Calculation
To perform a sample size calculation assuming you have significance level (α) and statistical power (1 - β), follow these steps:
Determine the Null and Alternative Hypotheses:
Null hypothesis (H0): e.g., Mean reduction in blood pressure is the same in both groups.
Alternative hypothesis (H1): e.g., Mean reductions differ.
Specify Key Factors:
Significance Level (α): Probability of Type I error (false positive).
Statistical Power (1 - β): Probability of correctly rejecting the null hypothesis when the alternative hypothesis is true.
Effect Size (Δ): The expected difference in treatment outcomes that you aim to detect.
Variability (σ²): The expected variability in your outcome measure.
Use the Appropriate Formula: For continuous outcomes, the function can be represented as:
f(α, β) = applied values of significance and power. This function helps to compute the total sample size needed. For binary outcomes, the formula becomes:
n_{group} = \frac{\pi_1(1 - \pi_1) + \pi_2(1 - \pi_2)}{(\pi_2 - \pi_1)^2} f(α, β) where (\pi_1) and (\pi_2) are the proportions in the two groups.
Calculate Required Sample Size:
Plug the values of α, β, Δ, and σ² into the formula to find the required sample size for each group. Adjust the sample size as needed based on the context of your trial.
Consider Practicalities:
Ensure that the calculated sample size is feasible ethically and logistically for your trial design.
Excessive sample size is unethical and inefficient.
Hypothesis Testing
Null hypothesis (H0): e.g., Mean reduction in blood pressure is the same in both groups.
Alternative hypothesis (H1): e.g., Mean reductions differ.
Types of Error
Type I error: False positive (reject H0 when true).
Type II error: False negative (fail to reject H0 when it should be).
Statistical Terms
Significance (Probability of type I error, α)
Power (Probability of rejecting H0 given that H1 is true, 1 - β).
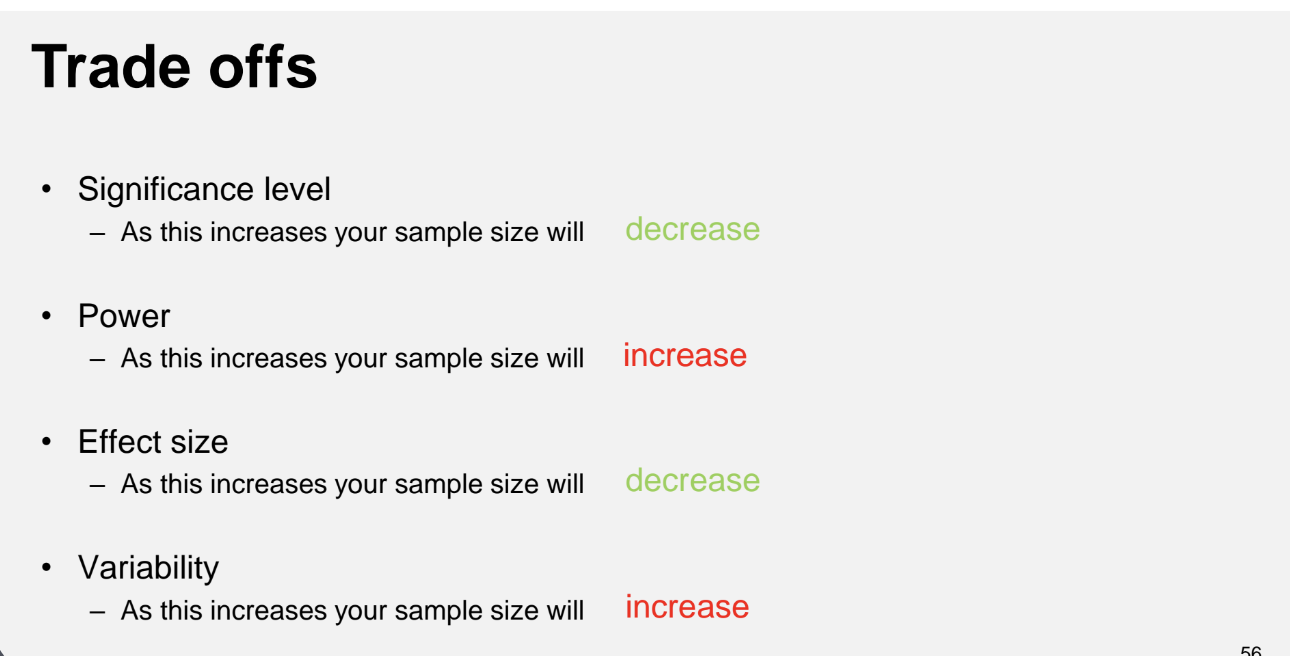
Key Factors Affecting Sample Size
Significance level (α)
Statistical Power (1 - β)
Effect size (Δ)
Variability (σ²)
Formula for Continuous Outcomes
Function f(α, β) = applied values of significance and power.
Example: PROMPT Study
Treatment: Two types of platelet transfusions.
Outcome: Platelet increments.
Sample Size for Binary Outcomes
Function for proportions in groups: [ n_{group} = \frac{\pi_1(1 - \pi_1) + \pi_2(1 - \pi_2)}{(\pi_2 - \pi_1)^2} f(α, β) ]
Summary
Explained randomisation methods to ensure treatment groups do not differ systematically.
Discussed stratification and minimisation to balance patient characteristics.
Emphasized the importance of blinding to reduce bias in clinical trials.
Detailed sample size calculations that account for effect size and variability.