Organic Chemistry
Alkanes
Some of the general features of alkanes are as follows:
Are the simplest organic compounds made up of carbon and hydrogen only
They have a general formula : C(n)H(2n +2)
Carbon atoms are bonded through a single covalent bond
They are also called saturated hydrocarbon
They contain strong carbon-to-carbon and carbon-to-hydrogen bonds hence they are chemically inert sometimes they are referred to as paraffin (little affinity)
In methane carbon forms a single bond with four hydrogen atoms, all the hydrogen and carbon bond angles are at 109.5° and methane has a tetrahedral structure.
The carbon-to-carbon and carbon-to-hydrogen bonds are formed by head-on overlapping of the sp3 hybrid orbital of carbon and 1s orbitals of hydrogen.
Methane is a gas found in coal mines and marshy places.
Isomerism is when compounds have the same molecular formula but different structural formulas hence they obtain different properties. They are known as structural isomers. Such structural isomers which are different from carbon atoms are known as chain isomers.
Alka group is obtained from alkanes by removal of one hydrogen atom.
Types of alkanes:
Primary: carbon attached to one other carbon atom.
Secondary: carbon attached to 2 other carbon atoms.
Tertiary: carbon attached to 3 other carbon atoms and
Quaternary: carbon attached to 4 other carbon atoms.
Methods of preparation
From unsaturated hydrocarbons:
Hydrogen gas adds to alkynes or alkenes in the presence of finely divided catalysts like palladium, platinum, or nickel to form alkanes.
These catalysts absorb dihydrogen gas on their surface and activate the hydrogen-to-hydrogen bond.
Palladium and platinum can catalyze the reaction at room temperature but relatively higher temperature and pressure are needed for nickel.
Example: 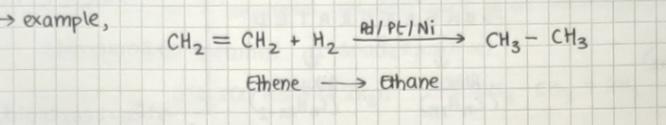
From alkyl halides:
Does not include fluoride as carbon and bonds are too strong.
Addition of hydrogen with zinc and hydrochloric acid gives alkanes
We use nascent hydrogen which is a very reactive form of hydrogen, it is also a good reducing agent.
Example:
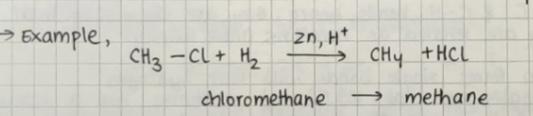
Wurtz reaction: alkyl halides on treatment with sodium metal in dry ether solution give higher alkanes
The reason we are using dry ether is that the intermediate formed during the reaction is a free radical and it reacts easily with hydrogen cation which is present in water.
Example: 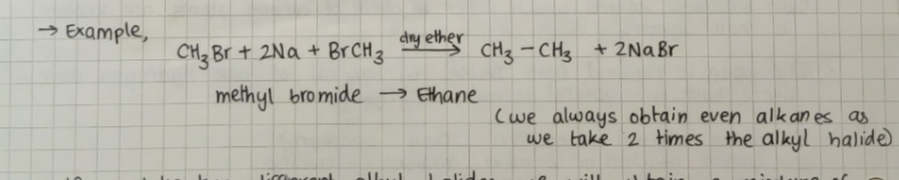
If we take two different alkyl halides we will obtain a mixture of three alkanes
Example: 
From carboxylic acids:
Elimination of carbon dioxide from carbolic acid is known as decarboxylation
Sodium salts of carboxylic acid on heating with soda lime give alkanes containing one carbon less than the carboxylic acid compound.
Example:
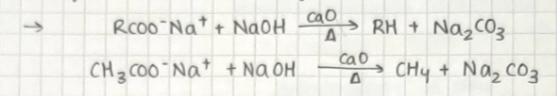
Kolbe's electrolytic method: here sodium or potassium aqueous solution salt of carbolic acid is electrolyzed. Alkanes are obtained at the anode.
Example: 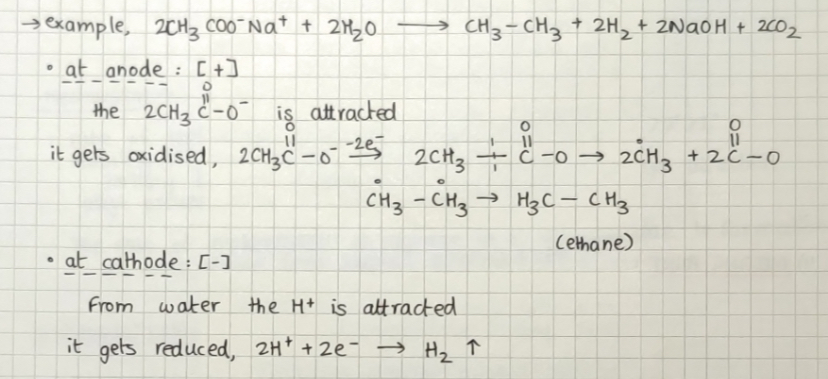
We cannot make methane using this method as alkane is formed by the combination of two alkyl radicals whereas methane only has one carbon atom.
Physical properties
Physical state:
Alkanes are nonpolar, hence they have london or dispersion intermolecular forces which are the weakest. Hence, they are in a gaseous state.
London forces increase as the size of atoms increases. Hence alkanes from C5 to C17 are in liquid state.
C18 onwards are solids.
Solubility:
In water, polar/ionic compounds dissolve but we know alkanes are nonpolar.
Like dissolves like, hence they dissolve with other non-polar reagents such as Benzene.
Boiling point:
They are also dependent on the magnitude of intermolecular attraction.
As the number of carbon atoms increases, the size of atoms increases, and the wan der Waals forces increase, thus boiling point Increases.
Isomeric alkane: As the extent of branching increases, the size decreases, hence the boiling point decreases.
Melting point :
In a solid state, the molecules have a tight-packed arrangement.
Even number alkanes are more symmetrically packed as they are structures. Hence, its melting point is a bit higher than expected. (Alternate effect)
Overall with an increase in the number of carbon atoms in the alkane, the melting point increases
Density:
As the molecular mass increases, the density increases.
After a certain point, the density becomes constant. This is the reason oil floats on water.
Chemical properties
Halogenation:
A hydrogen atom from the alkane is replaced by a halogen.
Chlorination - takes place in the presence of light/ UV rays
Bromination - is slow
Iodination - super slow almost reversible
Fluorination - too violent to be controlled
Combustion:
Alkanes on heating in the presence of air or dioxygen are completely oxidized to carbon dioxide and water with the evolution of large amounts of heat.
General equation -
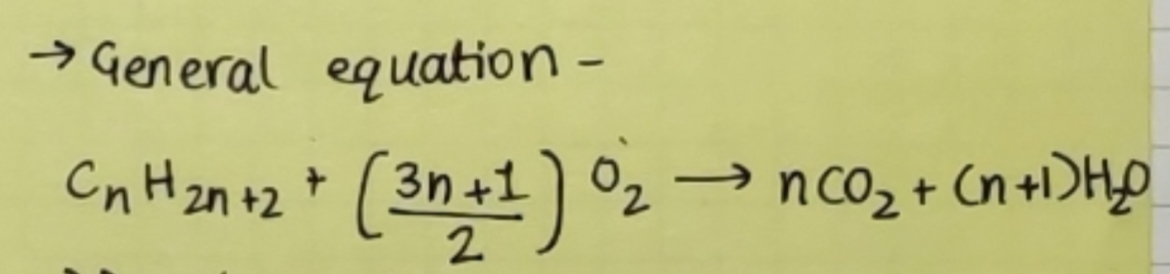
Due to the evolution of large amounts of heat during combustion, alkanes are used as fuels.
During incomplete combustion due to insufficient oxygen or dioxygen, carbon black is produced, it is used to manufacture ink, black pigments, etc.
Controlled oxidation:
Alkanes on heating with a regulated supply of dioxygen or air at high pressure and in the presence of a suitable catalyst give alcohols, aldehydes or acids
When an alkane having tertiary carbon is treated with a strong oxidizing agent such as KMnO4 then alcohol is obtained.
Isomerization:
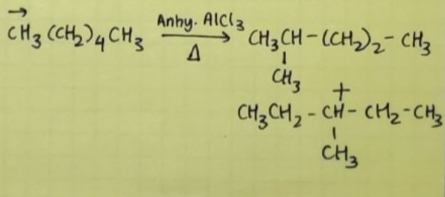
n-Alkanes on heating in the presence of anhydrous
aluminum chloride and hydrogen chloride gas isomerise to branched chain alkanes (majority)
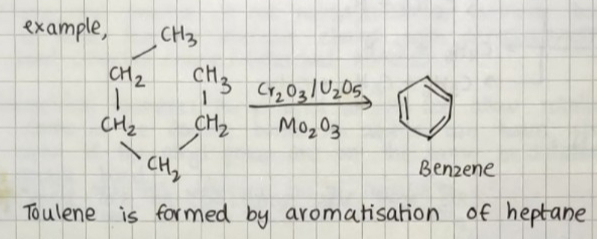
AROMATIZATION:
Process of converting aliphatic & acyclic hydrocarbons into aromatic hydrocarbons in the presence of suitable catalysts at high pressure & high temperature.
The two processes involved are-
Dehydrogenation
Cyclization
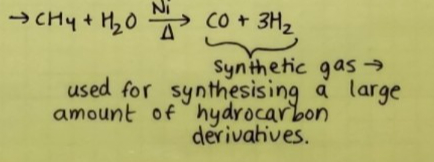
REACTION WITH STEAM:
Methane reacts with steam at 1273K in the presence of a nickel catalyst to form carbon monoxide & dihydrogen.
This process is used to produce H₂.
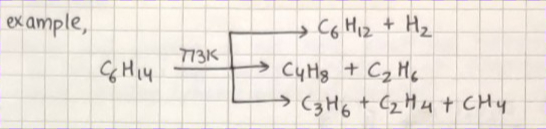
PYROLYSIS :
Higher alkanes on heating to higher temperatures decompose into lower alkanes, alkenes, etc.
Its a decomposition reaction, also called cracking.
It is believed to be a free radical reaction. Preparation of oil gas or petrol gas from kerosene oil or petrol involves the principle of pyrolysis.
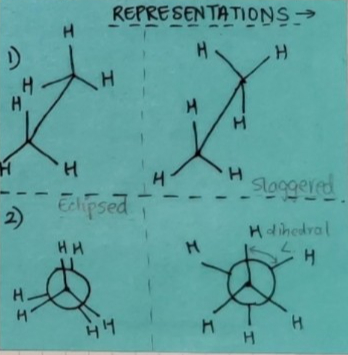
CONFORMATION:
Alkanes have a C-C sigma(σ) bond hence, rotation about the C-C bond is allowed. This rotation results in different spatial arrangements of atoms in space which can change into one another, such spatial arrangements are called conformers or conformations or rotamers.
Due to the weak repulsive interaction between the adjacent bonds the repulsive interaction is called TORSIONAL STRAIN.
TYPES OF CONFORMATIONS:
ECLIPSED: Hydrogen atoms attached to the carbon atoms are the closest.
STAGGERED: Hydrogen atoms attached to the carbon atoms are the farthest.
SKEW: any other intermediate conformation. From eclipsed to staggered.
There are infinite spatial arrangements in alkanes.
Conformational isomers: the spatial arrangement of hydrogen atoms attached to one carbon atom with respect to the other carbon atom.
REPRESENTATIONS OF CONFORMATIONS:
Sawhorse projections.
Newman projections.
Eclipsed is not as stable as staggered as it has the least torsional strain due to dihedral or torsional angle. Energy diff = 12.5 kJ/mol
Alkenes
Alkenes are hydrocarbons in which carbon has a double bond in their molecule.
They have a general formula - C(n)H(2n)
Alkenes are also known as olefins (oil forming). Ethene was found to form an oily liquid in reaction with chlorine.
In alkenes, carbon is sp^2 hybridized. Ethene is a planar molecule.
A pi bond is weaker than a sigma bond due to poor sideways overlapping between the 2p orbitals. Hence, alkenes are easily attacked by reagents or compounds which are short of electrons (electrophiles).
Ethene and propene do not have structural isomers but butene has 3 isomers. This is due to slight changes in the difference of position of the double bond (position isomer) / branched isomers.
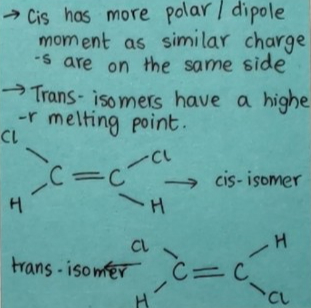
Geometrical isomers: The pi bond does not allow the rotation of the carbon atoms of the double bond. An alkene can have stereoisomers depending on the way the alkyl group is on the same or different sides of the double bond.
If they are on the same side then cis-isomers.
If they are on the opposite side then trans-isomers.
Due to different arrangements of atoms or groups in space, they have different dipole moments, melting points, solubility, etc.
Methods of preparation
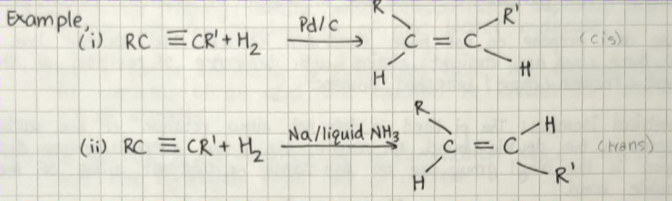
FROM ALKYNES:
Hydrogen has to be added in the presence of Lindlar's catalyst. Partial reduction of alkynes.
Lindlar's catalyst - Partially deactivated palletized charcoal.
Palladised charcoal - Palladium metal supported on charcoal. Partially deactivated by poison, such as quinoline, sulfur compounds.
Alkyne on reduction with the presence of Lindlar's catalyst obtains cis alkenes.
Alkynes on reduction with the presence of sodium with liquid ammonia gives trans alkenes.
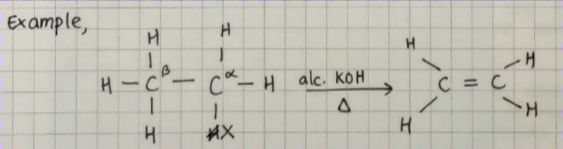
FROM ALKYL HALIDES :
Alkyl halides on heating with alcoholic potash (potassium hydroxide dissolved in alcohol) eliminate one molecule of halogen acid to form alkenes.
The process of removal of halogen acid (halogen + hydrogen) is known as dehydrohalogenation & it is an example of beta elimination.
The nature of the halogen atom and alkyl group determines the rate of reaction.
Iodine > Bromine > Chlorine
tertiary > secondary > primary
FROM VICINAL DIHALIDES :
Dihalides in which two halogen atoms are attached to two adjacent carbon atoms is known as vicinal dihalides.
Vicinal dihalides on treatment with zinc metal lose a molecule of ZnX2 to form alkene. This reaction is known as dehalogenation.
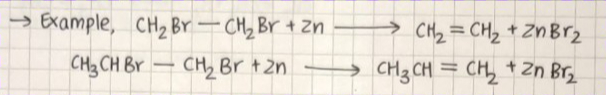
FROM ALCOHOLS BY ACIDIC DEHYDRATION:
Alcohols on heating with concentrated sulphuric acid form alkenes with the elimination of one water molecule.
Since a water molecule is eliminated from the alcohol molecule in the presence of an acid, the reaction is known as an acidic dehydration reaction.
This reaction is an example of beta elimination since the -OH group takes out one hydrogen from the beta carbon.
Physical properties
Higher alkenes - colorless and odorless
Ethene- colorless with the faint sweet scent
Alkenes are partially polar due to the pi bond
Are fairly soluble in nonpolar solvents such as benzene, ether, etc.
Weak Van der waals forces.
Chemical properties
ADDITION OF DIHYDROGEN :
Alkenes add up one molecule of dihydrogen gas in the prese
Alkanes.
Is known as hydrogenation → The metal catalyst breaks the bond between H2 & forms a weak metal-hydrogen bond which later breaks when H attaches to the alkene.
ADDITION OF HALOGENS :
Halogens like bromine or chlorine add up to alkene to form vicinal halides.
Iodine doesn't show this reaction as iodine is the least reactive halogen
The reddish-orange color of Br solution in CCl4 is discharged when Br adds up to an unsaturated site.
Hence, this reaction is used as a test for unsaturation.
This reaction is an example of an electrophilic addition reaction involving cyclic halonium ion formation.
ADDITION OF HYDROGEN HALIDES:
Hydrogen halides (HI> HBr>HCL) add up to alkenes to form alkyl halides, It is an example of an electrophilic reaction.
MARKOVNIKOV RULE »
"The negative part of the addendum (adding molecule) gets attached to that carbon atom which possesses a lesser number of hydrogen atoms.
ANTI MARKOVNIKOV RULE →
"In the presence of peroxide, the addition of HBr to unsymmetrical alkenes like propene takes place contrary to Markovnikov's rule.
ADDITION OF WATER :
In the presence of a few drops of concentrated sulphuric acid, alkenes react with water to form alcohols, in accordance with the Markovnikov rule.
OZONOLYSIS:
Involves the addition of ozone molecules to alkene to form ozonide. Cleavage of ozonide by Zn - H20.
Highly useful for detecting the position of double bonds in alkenes.
Alkynes
Are characterized by the presence of a triple bond in the molecule.
Their general formula is C(n)H(2n-2)
the most important members of this series of hydrocarbons and hence called acetylene
Far less stable compared to alkanes.
Ethyne and propene have one structure. Butyne has 2.
Methods of preparation:
FROM CALCIUM CARBIDE :
Limestone is heated and gives → Quick lime, then coke is added to give calcium carbide → Alkyne.
* We add water to calcium carbide.
FROM VICINAL DIHALIDES →
When we treat vicinal dihalide undergoes dehydrohalogenation with alcoholic potassium hydroxide
Physical properties:
The first 3 members are gasses. (C2- C4).
The next 8 liquids and C12 onwards are solids.
[As Van Der Waals forces increase ]
Higher alkynes are colorless and odorless
Ethyne is colorless but characterized by smell.
With the increase in size, the Boiling point increases.
Fairly soluble in polar solvents such as benzene, and ether.
Not soluble in water.
Density increases with an increase in size but is less denser than water
Chemical properties
ACID-BASE REACTION :
Sodium metal sodamide are strong base.
They react with ethyne to form sodium acetylide with liberation of dihydrogen.
ADDITION OF DIHYDROGEN :
Alkynes add up one molecule of dihydrogen in the presence of catalysts such as Nickel, Palladium & Platinum to form alkanes.
ADDITION OF HALOGEN :
Used as a test of saturation
ADDITION OF HALOGEN HALIDE :
Two halogen halide molecules (HI, HBr, HCL) are added to alkynes to give gem dihalides.
Gem dihalide: 2 halogens are attached to the SAME carbon.
REACTION WITH WATER :
one molecule of water adds to alkynes on warming with mercuric sulfate and dilute sulphuric acid at 333K, to form carbonyl compounds.
Arenes
Most of them possess pleasant odors.
Most such compounds are found to contain a benzene ring.
The benzene ring is highly unsaturated but in a majority of reactions retained.
There are aromatic compounds that do not contain a benzene ring but rather a highly unsaturated ring other than.
Aromatic compounds containing benzene ring are BENZENOID.
Those that do not contain benzene are NON-BENZENOID.
Benzenes molecular formula CH6 indicates a high degree of unsaturation.
But benzene was found to be a stable molecule and form a triozonide which indicates presence of three double bonds.
Benzene can form/ produce one and only one monosubstituted derivative, which indicates that all 6 carbon & hydrogen atoms are identical.
structure - carbon atoms with alternate double and single bonds with one hydrogen atom attached to each.
Benzene is stable due to the delocalization of its electrons, due to which they do not have a fixed position.
CONDITION FOR AROMATICITY →
(i) Should be planar
(ii) complete delocalization of pi electrons in the ring
Preparation
Cyclic polymerization is ethyne
Decarboxylation of aromatic acids
Reduction of phenol
Physical properties
Are non-polar
Colorless with characteristic aroma.
Burn with a sooty flame
Chemical properties
Nitration
halogenation (in the presence of Lewis acids)
Sulphonation
Friedel crafts acylation
Friedel crafts alkylation
Directive influence→ depends on the nature of the substituent already present in the ring
Ortho & Para directing:
• Seen in -OH, -NH2,- CH3, - CMs
• The electron density is more at the ortho & para position.
• Due to the -I effect, the electron density is reduced
• Activates the ring
Meta directing
• This group reduces density in the benzene ring due to high - I effect.
• Are also called deactivating groups.
Haloalkane and Haloarene
Our body produces iodine-containing carbon hormone thyroxine, a deficiency of which causes goiter.
Halothane is used as an anesthetic during surgery.
They act as starting materials for the synthesis of a wide range of organic compounds.
Also used as solvents for relatively non-polar compounds.
Haloalkane. → Replacement of hydrogen atom(s) in an aliphatic hydrocarbon by halogen atom (s)
Haloarene → Replacement of hydrogen atom (s) in an acyclic hydrocarbon by halogen atom (s) results in the formation of haloarene or aryl halide.
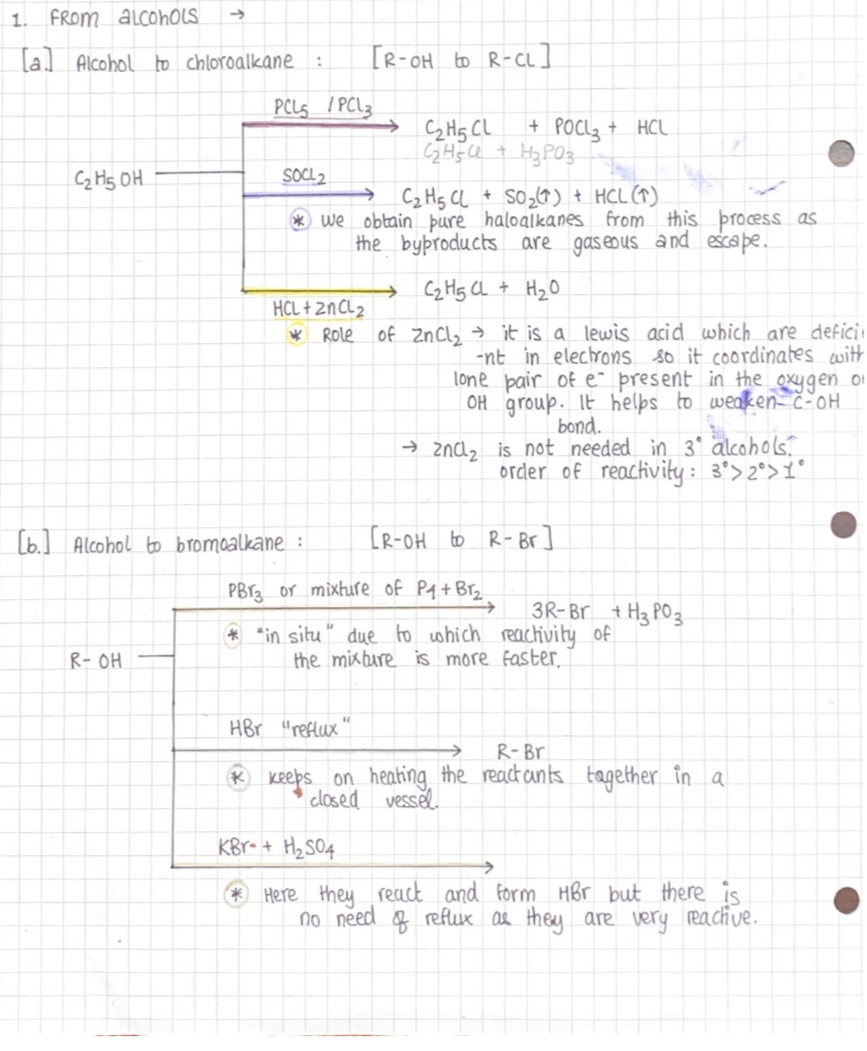
Methods of preparation
Preparation of haloalkane
From hydrocarbons→
From Alkanes:
A substitution reaction takes place in the presence of sunlight & halogen.
This process radical intermedal mechanism, which results in formation → order of stability: Benzylic > Allylic > 3° > 2°>1°
From alkenes →
An additional reaction takes place breaking the double bond on the addition of halogen halide.
Does not need sunlight.
We can also prepare dihalo compound
From halogen exhange:
For fluorine: swarts reaction
For iodine: Finkelstein reaction
Preparation of haloarene
By Benzene (direct halogenation)
It is an electrophilic- substitution reaction
To attack the benzene ring directly, we need an electrophile hence we use a Lewis acid which is electron deficient with the halogen to form a halogen cation that can attack the benzene ring.
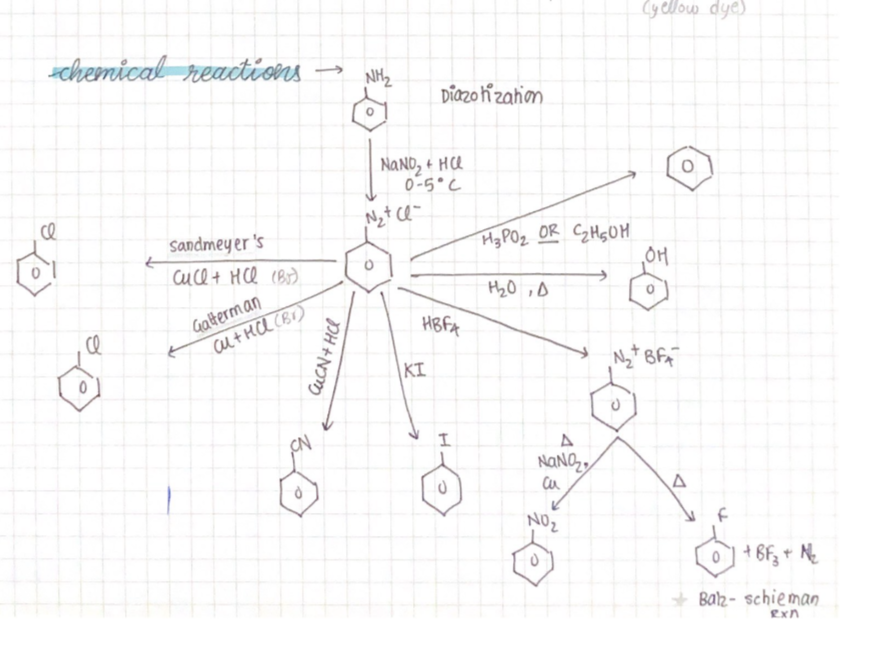
Sanmeyers reaction
When diazonium salt is treated with copper (I) chloride (Cu2Cl2) or copper (I) bromide (Cu2Br2), the corresponding haloarene is formed
Physical properties
In comparison to alkanes, haloalkanes are larger & polar.
They have high melting & boiling points.
haloalkanes are in a liquid state compared to their alkane which are in a gaseous state. Hence, the atoms are more associated.
melting point : R-F<R-CL <R-Br <R- I
Branching leads to a decrease in melting point as the size of the molecule increases, hence van der Waals forces decrease, and therefore melting point decreases.
Bromo, iodo & polychloro derivatives of hydrocarbons are heavier than water.
As the number of C or X atoms increases, the mass increases.
Chemical properties
Can react with metals - Wurtz reaction
Elimination reactions
Nucleophilic substitution reactions
Chemical properties of haloarene :
Aryl halides are extremely less reactive towards nucleophilic
substitution reaction:
Resonance effect: The c-X bond attains a double bond character due to resonating structures that require more energy to be broken.
Hybridization: carbon in haloarene. it is spa hybridized sa, whereas in Haloalkane
Cation: The phenyl carbon is not stabilized by resonance
Election rich: Arenes do not lack electrons and hence will end up
repelling approaching rich nucleophiles.
Stereochemistry :
Stereoisomers: compounds having the same molecular formula & structure but different spatial arrangement.
Types :
Optical
Conformational
Geometrical
Aldehyde and ketone
Aldehyde: The carbonyl group is attached to one hydrogen atom and one alkyl or aryl group.
Ketone: The carbonyl group is attached to two alkyl/aryl groups
Preparation of aldehyde and ketone
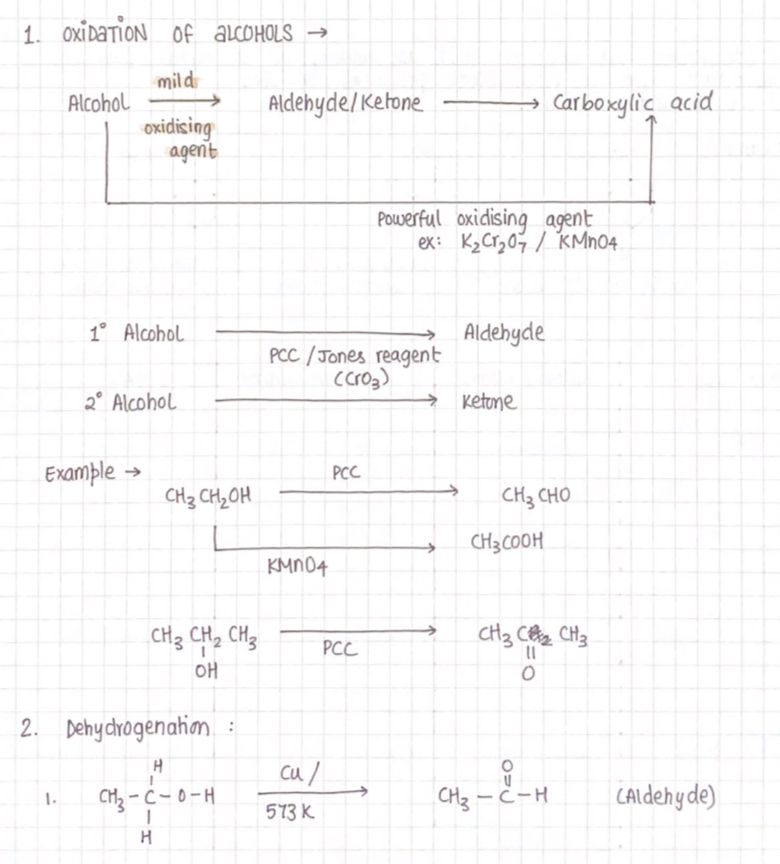
3. Hydration of alkynes
Acetylene on hydration gives aldehydes, other alkynes give ketones.
Preparation specifically for aldehyde:
Rosenmund reaction
Etard reaction
Stephen reaction
Gatterman Koch reaction
Preparation specifically for ketones:
Acyl chlorides
Friedel craft acylation
Nitrites
Physical properties
• The boiling point of aldehyde, ketone is higher than other hydrocarbons and ethers.
Due to weak molecular associations in aldehyde & ketone arising
out of the dipole-dipole interactions.
However, their boiling point is lower than alcohols of comparable mass due to the absence of intermolecular hydrogen bonding.
Alcohol > Ketone > Aldehyde > Alkane
• Lower members are miscible, but on increase in the alkyl group, miscibility decreases due to the hydrophobic part increasing.
• Higher aldehydes have a fragrant smell and lower have sharp, pungent odors.
Chemical properties
They undergo nucleophilic addition reactions due to the polar bond, Oxygen is more electronegative and due to the pi bond, carbon becomes the electrophilic center
Carboxylic Acid
Preparation
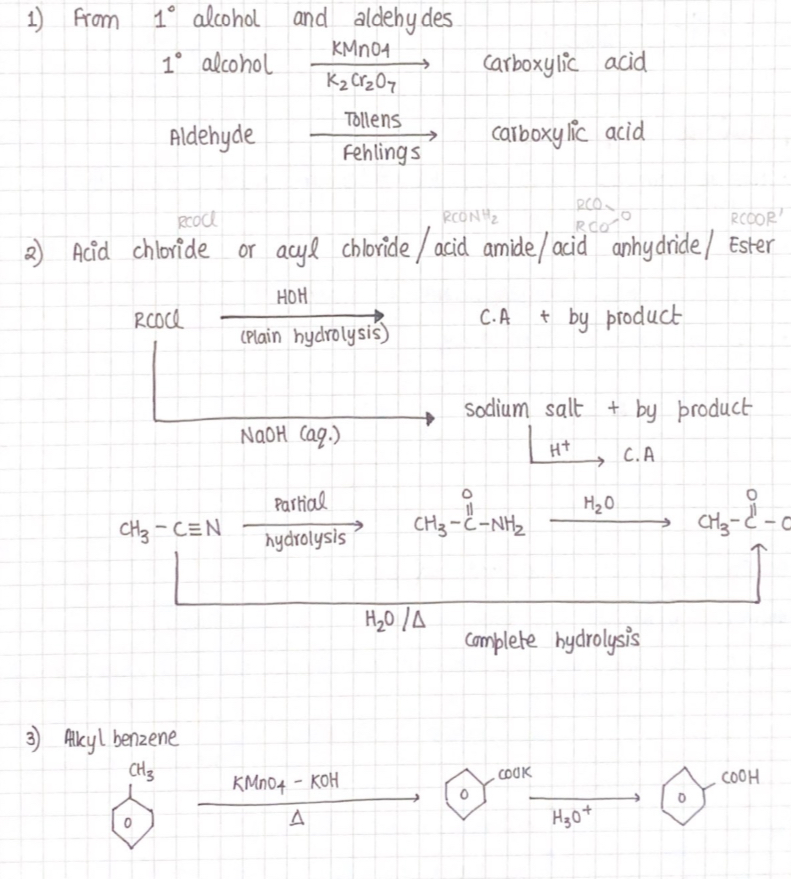
Physical properties
• None of them exist as a gas, due to intermolecular H-bond and extensive bonding.
• Low volatility
• higher boiling point than aldehyde, ketone, and alcohols.
• Exist as a dimer in the vapor phase
Chemical properties
They are good acids because the O-H bond is polar and H is acidic. Give resonance-stabilised carboxylate anions and hydronium ions.
Can undergo:
Decarboxylation
Halogenation
Ring substitution
Alcohol and Phenol
Alcohols: are formed when an H atom in aliphatic hydrocarbon (straight chain) is replaced by -OH.
Phenols are formed when an H atom in aromatic hydrocarbon (ring hydrocarbon, is replaced by -OH.
Ethers are formed when the H atom in a hydrocarbon is replaced by an alkoxy or aryloxy group
Uses :
Ethanol is used in sanitizer.
Spirit - nail polish remover
methoxymethane is an effective pain-relieving drug.
An alcohol contains one or more hydroxyl (OH) group(s) directly attached to carbon atoms of an aliphatic system.
Alcohol is less acidic than water.
WHEREAS phenols are more acidic than water.
Preparation of alcohol
From alkene :
Acid-catalyzed reaction:
Alkenes react with water in the presence of acid as a catalyst
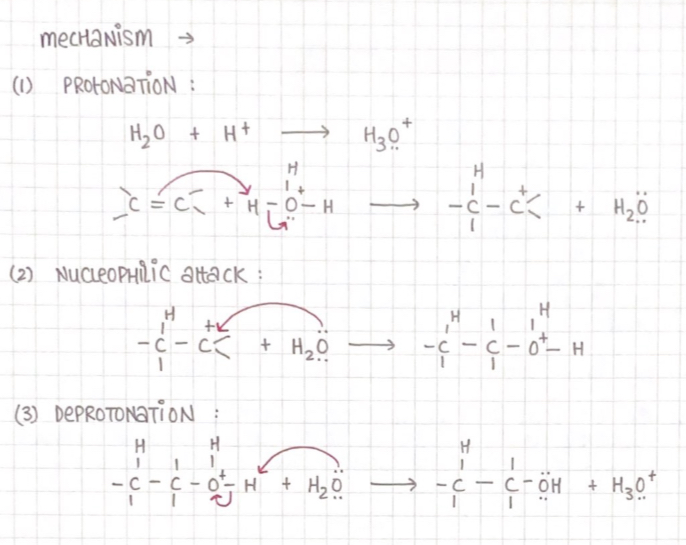
From amines
From the reduction of aldehyde or ketone
From the reduction of carboxylic acids
Reduction of esters
Grignard's reagent
Preparation of phenol
From haloarene :
Chlorobenzene is fused with NaOH at 623k and 320 atm which gives sodium phenoxide which on acidification gives phenol. (Dow's process)
From benzene sulphonic acid :
Benzene is sulfonated with oleum & benzene sulphonic acid so formed is converted into sodium phenoxide on heating with molten sodium hydroxide.
From diazonium salt
From cumene
Physical properties of alcohol
• Lower alcohols are colorless at room temperature & are liquids.
• Smell & Taste :
Lower alcohols have a pleasant smell & burning taste.
Intermediate ones have an unpleasant smell.
Higher members are colorless and odorless & tasteless.
• The boiling point of lower alcohols is higher in comparison to other classes of compounds. This is due to intermolecular Hydrogen bonding.
• BP increases with an increase in molecular mass due to an increase in Vander Waals force.
• BP decreases with an increase in branching as the surface area decreases due to which VanderWaals forces decrease.
• Alcohols are soluble in water due to their ability to form hydrogen bonds
Physical properties of phenol
• Have higher BP in comparison to alcohol & comparable mass alkane due to intermolecular H bond.
• BP decreases with branching but increases with an increase in molecular mass.
• Are soluble in water.
• Solubility decreases with an increase in aryl groups as they are hydrophobic.
Chemical properties
Alcohols behave as nucleophiles when the O-H bond is broken.
The lone pair on o makes it nucleophile.
Shows the acidic behavior of alcohol.

2. Esterification
3. Acetylation
Reactions where C-O bond breaks :
Phenols do not show these reactions due to the presence of a partial double bond
But for alcohol:

Reactions :
Nitration
Halogenation
Reaction with zinc dust
Kolbe's reaction
Oxidation
Ethers
is an important solvent for paints, perfumes, oils, etc.
is used in the pain-relieving drug (codeine- methoxymethane)
Anesthesia - dimethyl ether
Ethers are formed when the H atom in a hydrocarbon is replaced by an alkoxy or aryloxy group.
Preparation
Acidic dehydration of alcohol
Alcohols undergo dehydration in the presence of protic acid but only at 443K temperature
Williamson synthesis
From Phenols
Physical property
Dimethyl ether & diethyl ether are gasses while others are colorless liquids.
Have a pleasant smell.
Are lighter
Are soluble in water, as they can form hydrogen bond
Are volatile & inflammable
The boiling point with an increase in mass increases, due to van der Waals forces increases.
The C-O bond is ether polar, thus ethers have a net dipole moment. The lone pair present in o causes the structure to be bent due to lone pair - lone pair repulsion, which causes the addition of dipole.
Chemical property
Is the least reactive group as it is slightly polar only
They react at high temperatures and with only excess hydrogen halide
Amines
Amines are derivatives of ammonia in which one or more hydrogen atom is replaced by an alkyl group.
Amines can be classified into primary, secondary or tertiary depending upon how many hydrogen atoms from ammonia are replaced.
Preparation
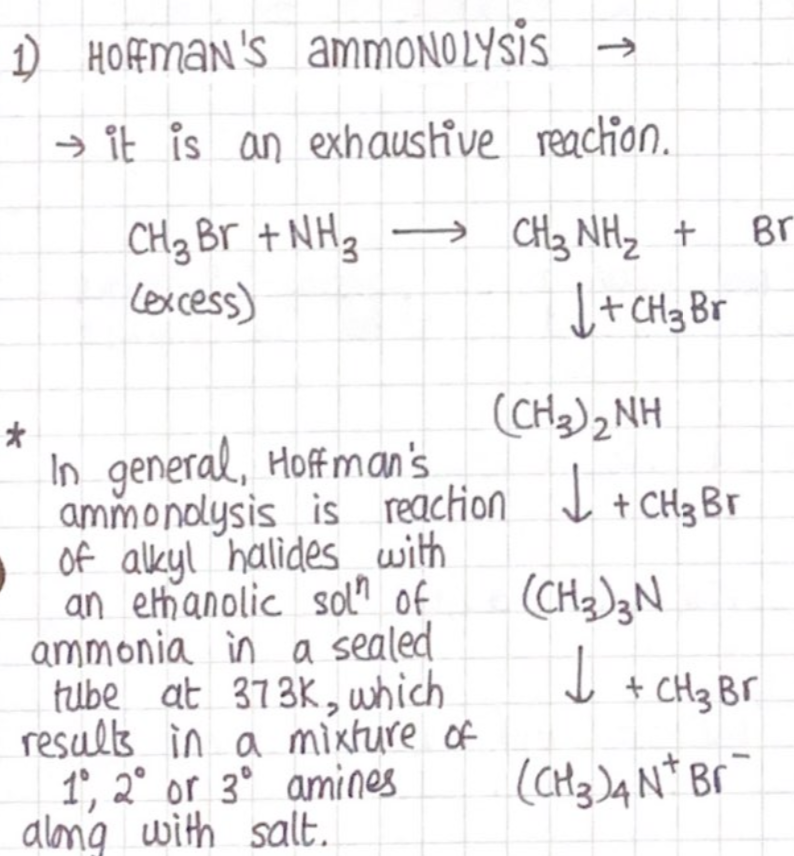
Reduction of nitro compounds
Reduction of nitriles
reduction of amides
Gabriel phthalimide synthesiS
Chemical properties
Amines are reactive due to:
The presence of an unshared pair of electrons on the nitrogen
The n-H bond is polar due to the difference in electronegativity.
The following reactions take place with corresponding regents:
 Knowt
Knowt