L11: Instrumentation for Fluorescence spectroscopy
Instrumentation for fluorescence
Filter cube
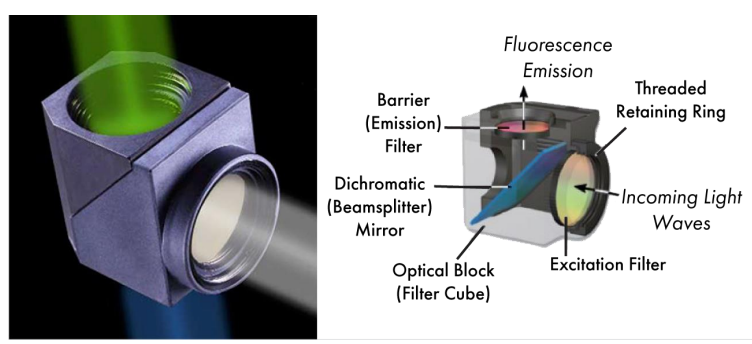
dichromatic mirror - only transmits light above a certain wavelength
Inner filter effect
light is absorbed by a material, which reduces the intensity of emitted light
high concentration - absorbs more than emits
can impact the accuracy of techniques like fluorescence spectroscopy
minimized by diluting the sample.
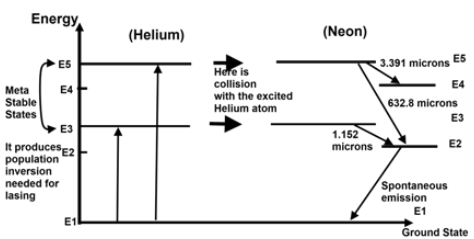
Stimulated emission and LASER
Light Amplification by Stimulated Emission of Radiation
Spontaneous vs stimulated emission
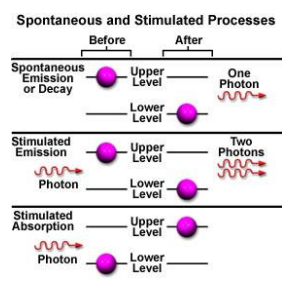
Stimulated emission is
Monochromatic (one colour/wavelength/energy)
Directional (perpendicular to excitation)
Polarized (travel in same plane)
Coherent (release at same time, travel in phase)
Fluorescence Spectrometer
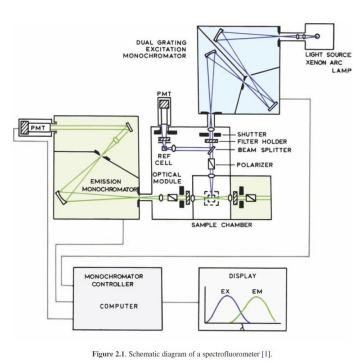
Excitation source (lasers, xenon arc lamps, LEDs)
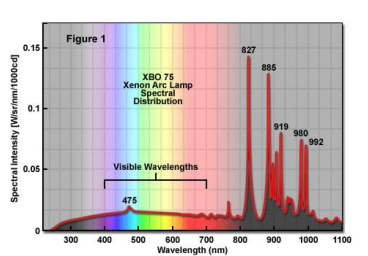
Xenon arc lamps (broad spectrum, general use)
Lasers (high intensity, monochromatic, ideal for single-molecule studies)
LEDs (very common, cheap, stable, useful for portable sensors)
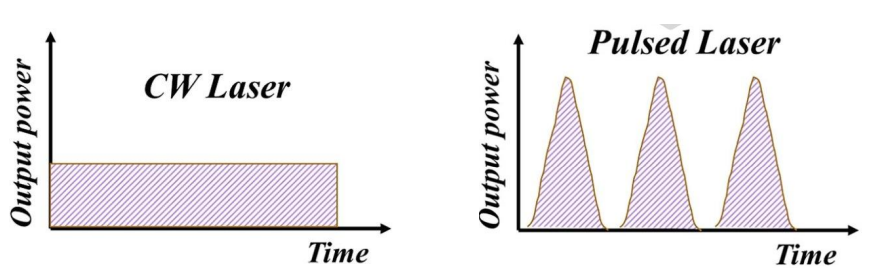
pulse width - structural or even quantum properties of a system - cyclic excitation
photochemistry - adjust pulse width to reaction time to
Excitation monochromator/filter (selects excitation wavelength)
extract specific wavelengths from broad-spectrum composite light source, i.e. prism
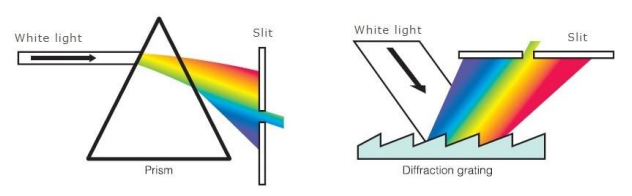
diffraction grating - smooth material with indented patterns; different wavelengths split/bend at different angles
Sample chamber (holds fluorophore or biomolecule)
90° from excitation direction
absorbance spectra - stops here
Emission monochromator/filter (selects emitted wavelength)
measure all wavelengths one at a time
Detector (PMT, CCD, or avalanche photodiodes for lowlight detection)
historically - eye
photo multiplier tubes - produce electrons in response to photons
spectrum - wavelength vs. # of photons
Importance
high sensitivity - look at things which are active
molecular specificity - molecular beacons (fluorophores, dyes, fluorescent proteins) = macromolecules or small molecules which you can attach to your biological molecule of interest AT SPECIFIC LOCATIONS
temporal dynamics from about 100 microseconds all the way to seconds
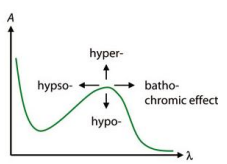
Spectral shifts
observed upon changes in solvent environment
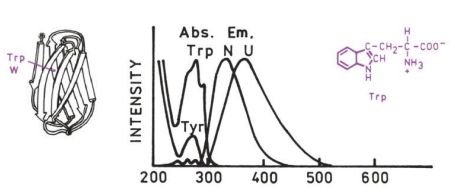
Applications in biochemistry
sensing, tracking, reaction
Proteins
location
conformation
open/closed
folded/unfolded
binding
Chemical sensing of the environment
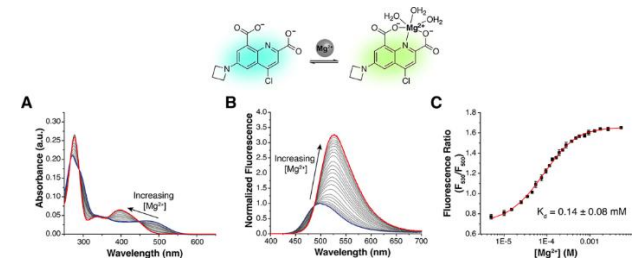
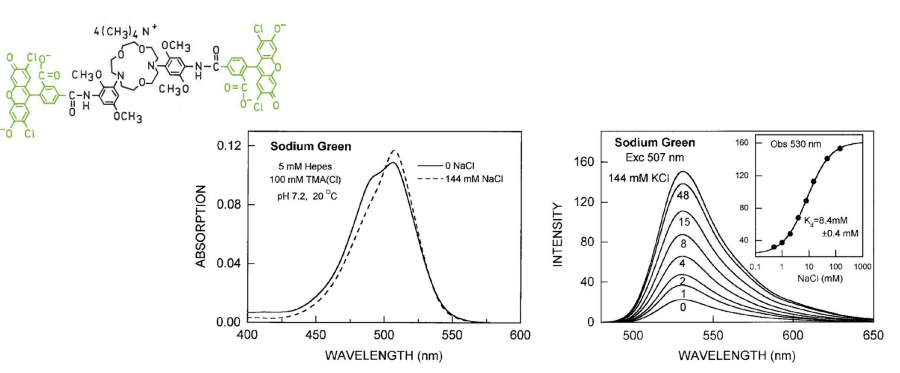
Kinetics
localization studies or binding studies also as a function of time
Physical sensing
dying cell - enzymes fall off
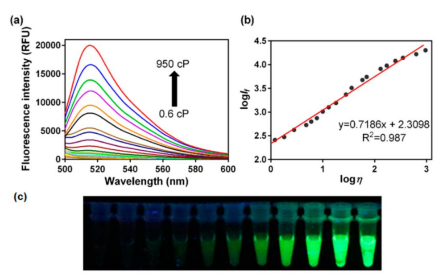
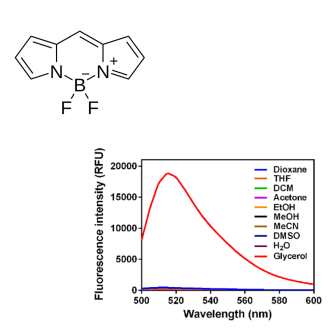
Advanced applications
Chronic pain is a huge burden on global health.
Sodium channels regulate pain through poorly known pathways (Nav1.7, Nav1.8, Nav1.9, etc)
sodium channels are too active - chronic pain
sodium chennels aren’t active enough - CIPA
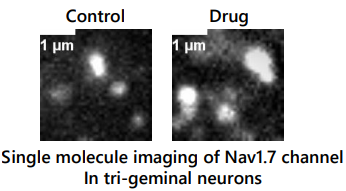
Why do Nav1.7 molecules cluster? Are there other pain signaling proteins in the cluster?
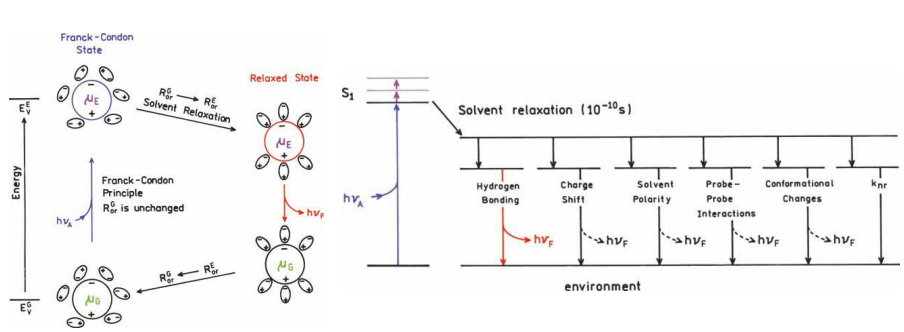
Why is there energy loss before fluorescence?
Energy loss is due to a variety of dynamic processes that occur following light absorption
The excess vibrational energy is rapidly lost to the solvent.
If the fluorophore is excited to the second singlet state (S2), it rapidly decays to the S1 state in 10–12 s due to internal conversion.
Solvent effects shift the emission to still lower energy due to stabilization of the excited state by the polar solvent molecules.
Solvent relaxation
Rotational motions of small molecules in fluid solution are rapid, typically occurring on a timescale of 40 ps or less.
Fluorescence lifetime is ns or more
This allows for the solvent molecules to reorient around the excited-state dipole, which lowers its energy and shifts the emission to longer wavelengths.
dipole moment - not the same in ground and excited
different attractions
different energy - minimisation
This occurs within 10–10 s in fluid solution.
very sensitive - can result in substantial Stokes shifts.
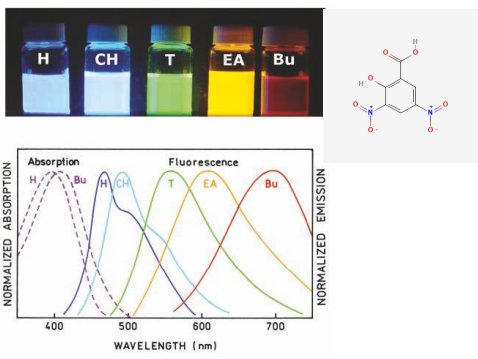
Other solvent factors
Solvent polarity and viscosity
Rate of solvent relaxation
Probe conformational changes
Rigidity of the local environment
Internal charge transfer
Proton transfer and excited state reactions
Probe–probe interactions (collision)
Changes in radiative and non-radiative decay rates (light intensity)
 Knowt
Knowt